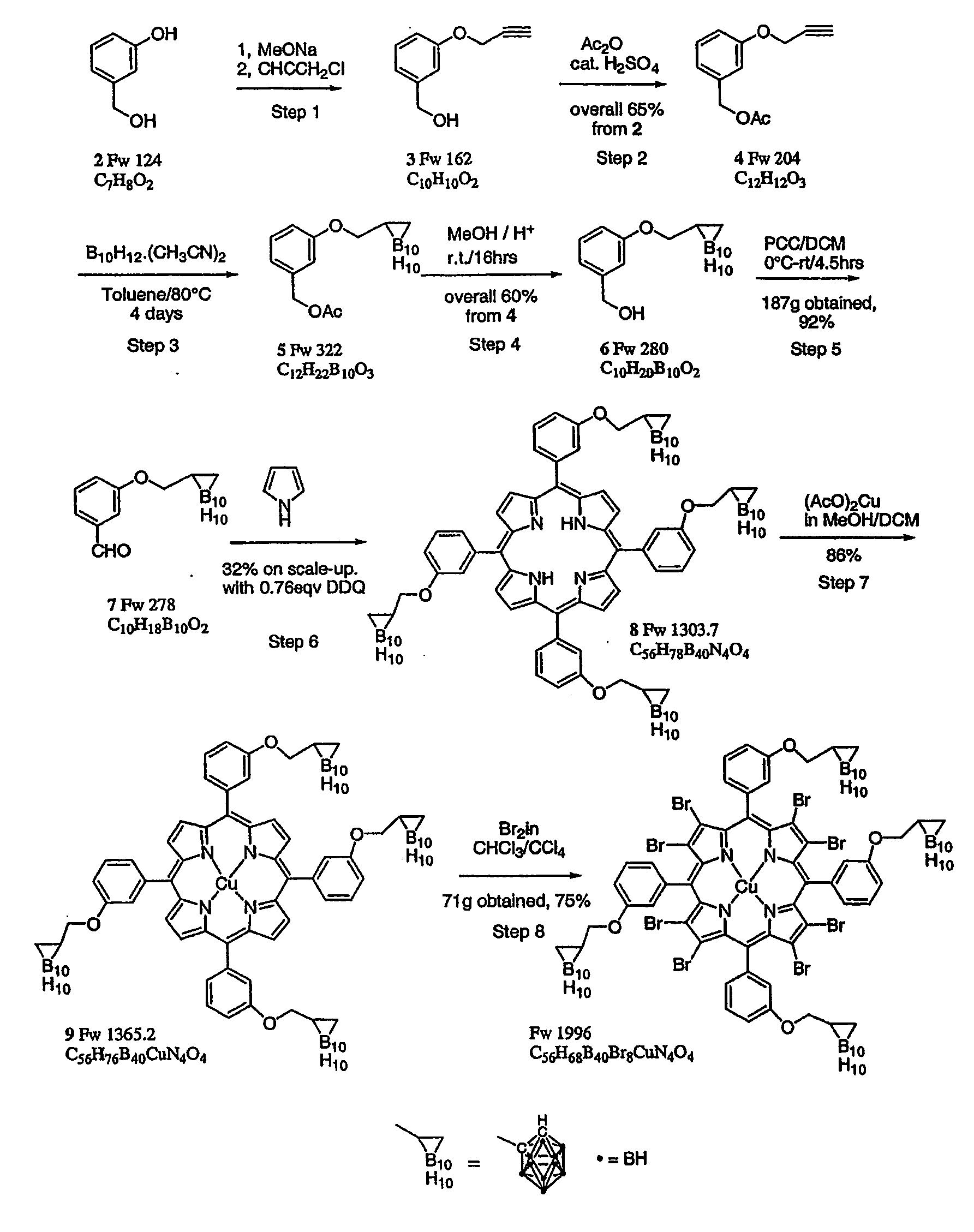
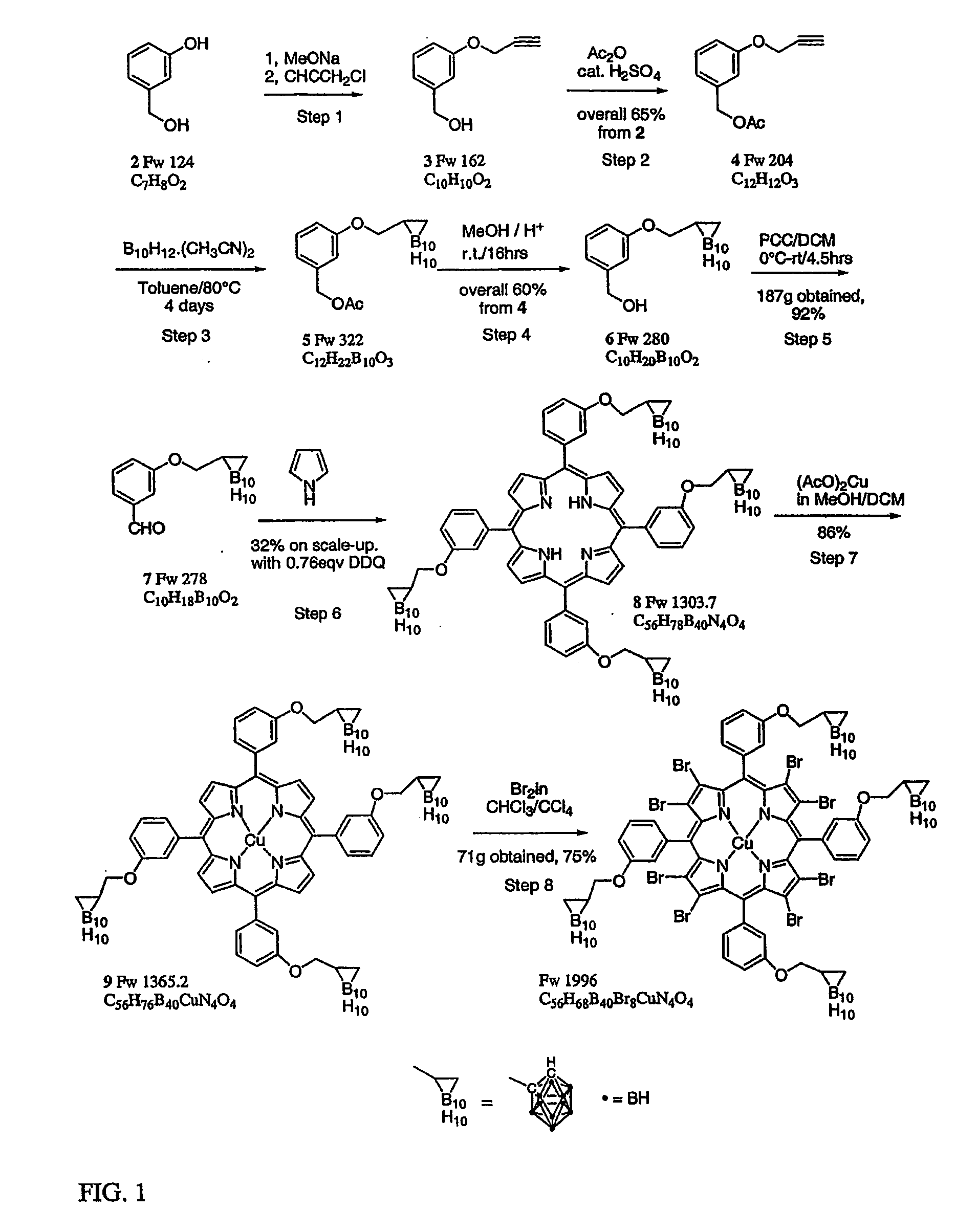
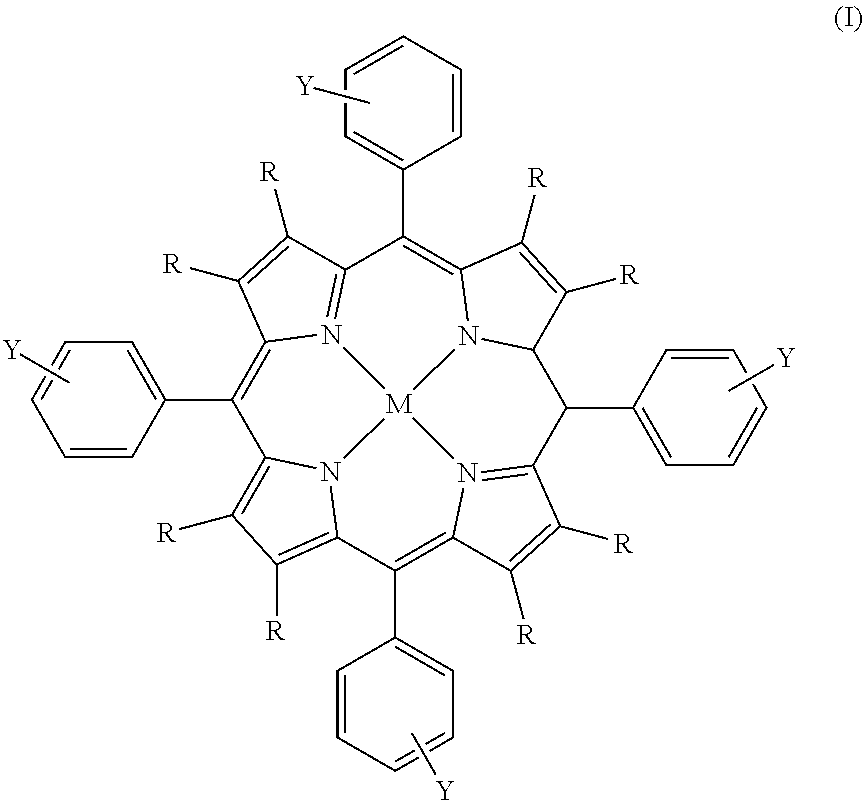
Process for the preparation of a boron-substituted porphyrin
a technology of porphyrin and boron, which is applied in the field of porphyrin production, can solve the problems and inability to achieve the effect of normal tissue damage and burdensome treatment schedule,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0063]All reactions were carried out under a nitrogen atmosphere in high temperature oven-dried glassware, with magnetic stirring or overhead stirrer unless otherwise stated. All intermediates and products were identified by means of proton NMR (where possible), TLC and MALDI TOF mass spectroscopy (in a dithranol matrix).
Preparation of 3-propargyloxybenzyl alcohol 3
[0064]
[0065]A 20.0 L flange flask was charged with 3-hydroxybenzyl alcohol (97%, ex. Aldrich, 903 g, 7.28 mol, 1 eq.) in methanol (MeOH)(9.1 L, dried over 3 A molecular sieves) to give a clear solution, into which sodium methoxide (25% in methanol, ex. Aldrich, 1.746 L, 7.63 mol, 1.05 eq.) was slowly added with vigorous stirring. After stirring for 10 min. the propargyl chloride (70 wt % in toluene, ex. Aldrich, 542.6 g, 805 ml, 7.29 mol, 1 eq) was added slowly with vigorous stirring over a period of 30 minutes. The reaction mixture was heated under reflux for 40 h (isomantle set to 76° C. with an internal temp. of 64° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com