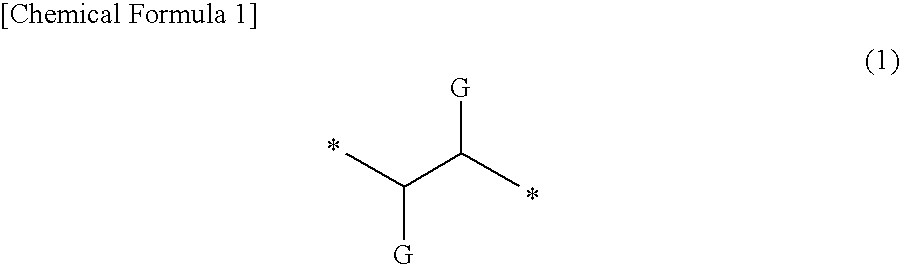
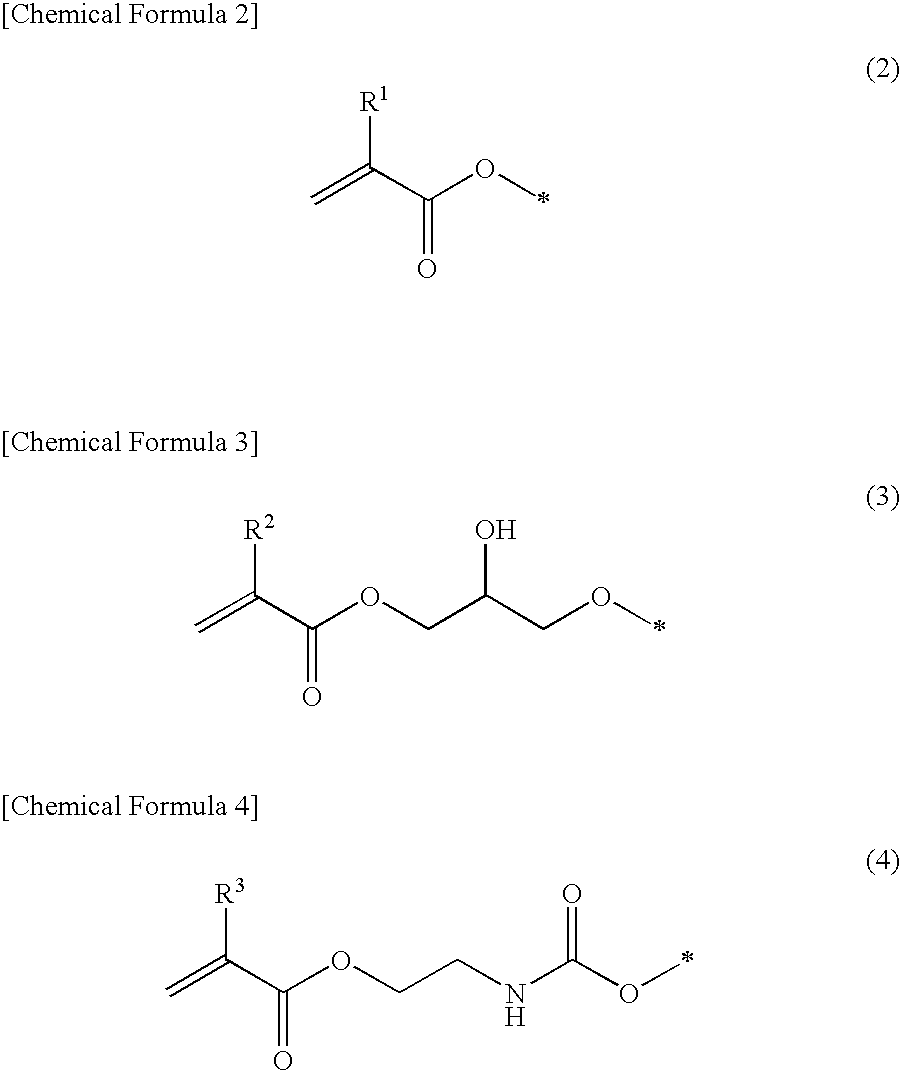
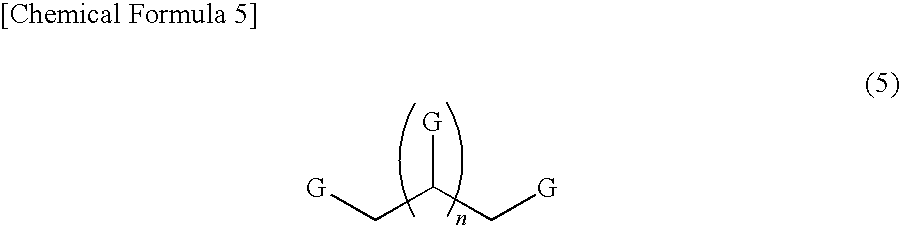
Polymerizable monomer-containing composition
a polymerizable monomer and composition technology, applied in dental prosthetics, impression caps, coatings, etc., can solve problems such as reducing bond strength, and achieve excellent curability and adhesive properties, high bond strength and bond durability, and high penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Application to One-Step Bonding System (One-Component Bonding Material)
(1) Production of One-Component Bonding Material
[0159]Respective components were mixed together at an ordinary temperature and thereby a one-component bonding material composition was produced. The composition thereof is indicated in Table 1. The bond strength with respect to bovine teeth dentin was measured according to the following procedure.
TABLE 1One-Component Bonding Material Compositions and Bonding Evaluation ResultsEx.Ex.Ex.Ex.Ex.Ex.Ex.C Ex.C. Ex.C. Ex.C. Ex.C. Ex.C. Ex.C. Ex.Components1-11-21-31-41-51-61-71-11-21-31-41-51-61-7PolymerizableEDMA3030153030monomer (A)XDMA30SDMA30MDMA303015Polymerizable#80130monomer forGDMA3030comparisonErMA30with (A)Trimethylol-30propane tri-methacrylateWater (B)Distilled water15151515151515215001515151515PolymerizableMDP1010101010101010101010101010monomer (C)having acidicgroupCrosslinkableBis-GMA3030303030303030303030303030polymerizablemonomer (D)Solvent (E)Ethanol15151515...
example 2
Application to Two-Step Bonding System (Two-Component Bonding Material)
(1) Production of Primer Using Polymerizable Composition Containing Polymerizable Monomer (A)
[0172]The respective components were mixed together at ordinary temperature and thereby primer compositions were produced. The compositions thereof are indicated in Table 2.
TABLE 2Primer Compositions and Bonding Evaluation ResultsEx.Ex.Ex.Ex.Ex.Ex.Ex.C. Ex.C. Ex.C. Ex.C. Ex.Components2-12-22-32-42-52-62-72-12-22-32-4PlymerizableEDMA3535703535monomer (A)XDMA35SDMA35MDMA3535PolymerizableGDMA3535monomer forErMA35comparisonwith (A)Water (B)Distilled water50505050505050315005050PolymerizableMDP2020202020202020202020monomer (C)having acidicgroupCrosslinkable#8011010101010101010101010polymerizablemonomer (D)Solvent (E)Ethanol5050505050505095505050PolymerizationTMDPO0.80.80.80.80.80.80.80.80.80.8initiator (F)CQ0.8PolymerizationDBB1accelerator (G)Ratio of water (B) with respect77777777775050523077777to 100 parts by weight of thewh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean particle size | aaaaa | aaaaa |
| mean particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com