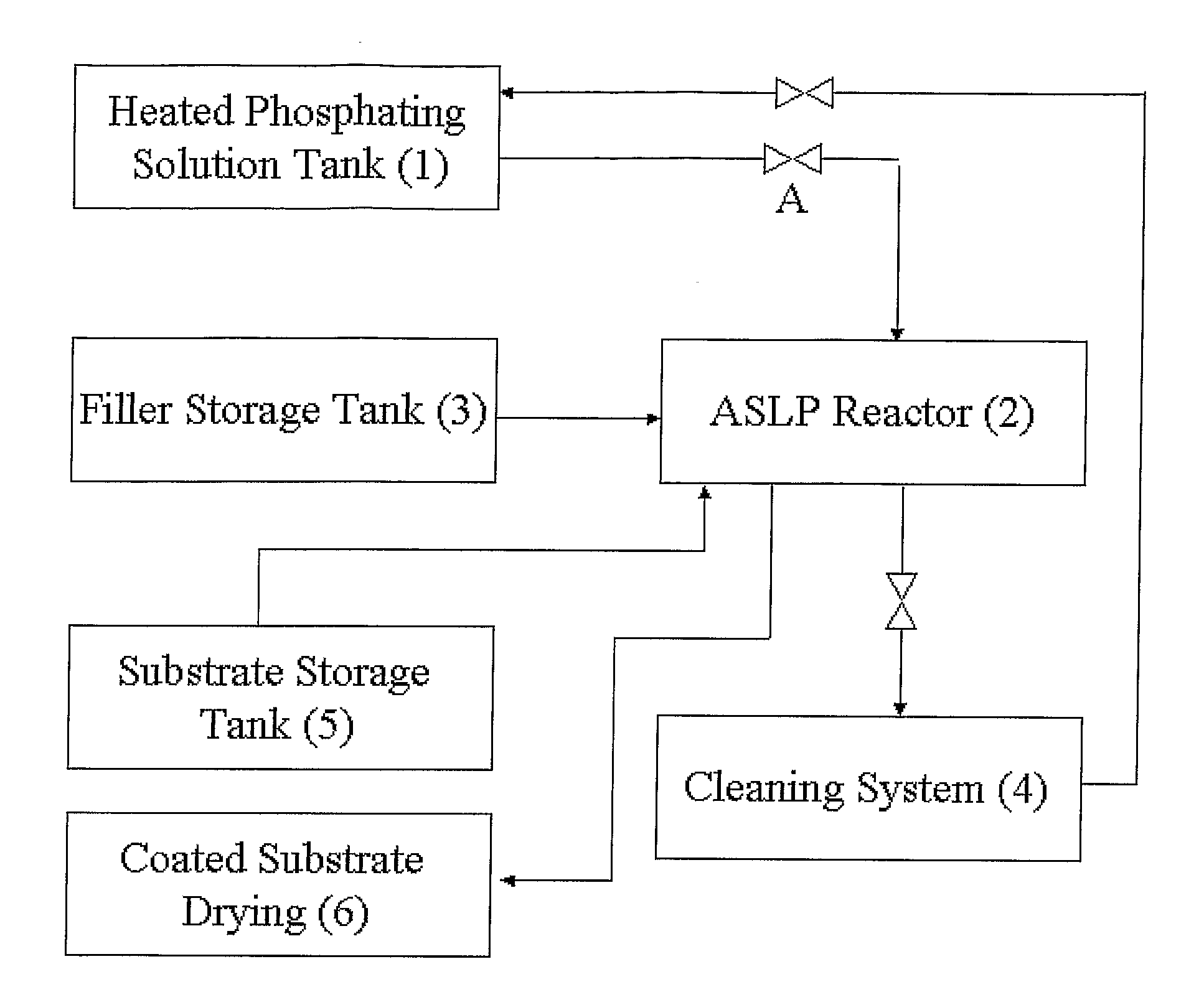
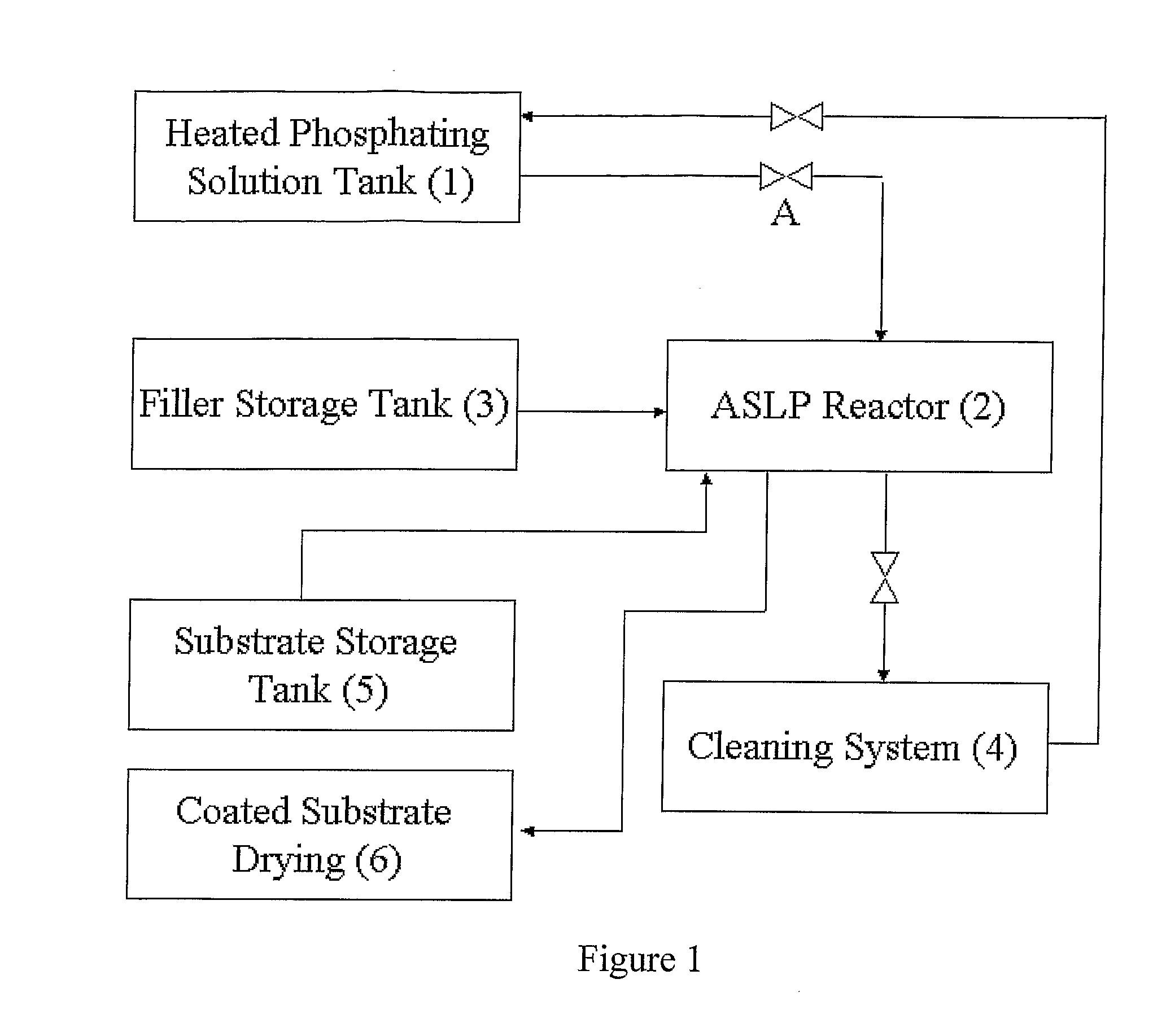
Method of applying zinc-phosphate conversion crystal coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment # 1
Experiment #1:
[0100]1. 300 ml of solution, prepared as described above, was added to a plastic beaker, having a volume of 0.5 liter.[0101]2. The quantity of metal for phosphating was 100 g.
experiment # 2
Experiment #2:
[0102]1. 700 g of porcelain chips, having triple-edged prism shape, with sides 3*3*4 mm and height 4 mm, was added to a plastic drum.[0103]2. 300 ml. of solution was added to the drum, covered with a lid, and was rotated at a rotation speed of 2 rpm, in a horizontal position during 5 minutes.[0104]3. The lid was then removed and solution fully removed, except the solution absorbed on the filler particle surfaces.[0105]4. The results of weighting show that in the drum, about 28 ml of phosphating solution was absorbed by chips and drum' sides.[0106]5. 300 g of metal for phosphating, including three control samples, were added to the drum.[0107]6. The drum was closed and revolved with rate 0.3 rpm during 10 minutes.
experiment # 3
Experiment #3: Repeated Experiment #2 with the difference being that the rate of revolution during phosphating process was 2 rpm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com