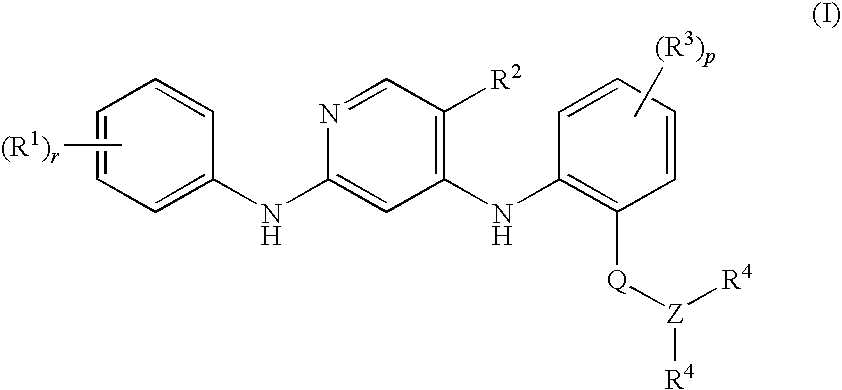
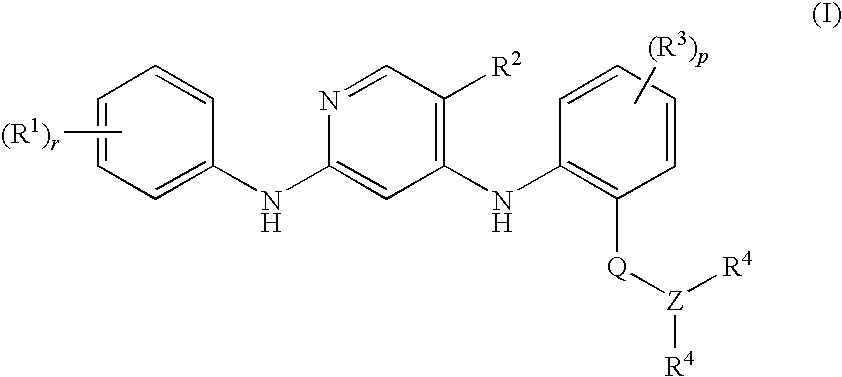
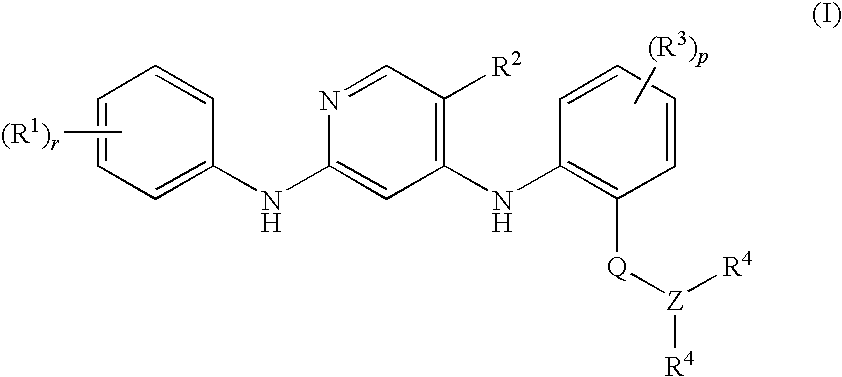
Anilinopyridines as inhibitors of fak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106]N-Methyl-2-{[2-[2-oxo-2.3-dihydro-1H-indol-5-yl)amino]-5-(trifluoromethyl)-4-pyridinyl]amino}benzamide
[0107]2-{[2-Chloro-5-(trifluoromethyl)-4-pyridinyl]amino}-N-methylbenzamide (Intermediate 1, 50 mg, 0.152 mmol), 5-amino-1,3-dihydro-2H-indol-2-one (90 mg, 0.607 mmol), 1.0 M hydrochloric acid (0.3 mL) 1,4-dioxane (1.0 mL), and water (1.0 mL) were added to a vessel, which was sealed and heated at 150° C. for 2 days. The resulting crude material was purified by reverse-phase HPLC to give the title compound as a solid (20 mg, 26% yield). MS: M(C22H18F3N5O2)=441.41, (M+H)+=442; 1H NMR (400 MHz, DMSO-d6) δ ppm 2.76 (d, J=4.55 Hz, 3 H) 3.45 (s, 2 H) 6.68 (s, 1 H) 6.74 (d, J=8.34 Hz, 1 H) 7.11 (dd, J=15.03, 1.14 Hz, 1H) 7.26 (dd, J=8.46, 2.15 Hz, 1 H) 7.43-7.52 (m, 1 H) 7.54-7.60 (m, 1 H) 7.71 (dd, J=7.83, 1.26 Hz, 1 H) 8.18 (s, 1 H) 8.23 (s, 1 H) 8.67 (d, J=4.55 Hz, 1H) 9.07(s, 1 H) 10.15 (s, 1 H) 10.24 (s, 1 H).
example 2
[0108]N-Methyl-2-{[2-{[4-(4-morpholinyl)phenyl]amino}-5-(trifluoromethyl)-4-pyridinyl]amino}benzamide
[0109]A microwave tube was charged with 2-{[2-chloro-5-(trifluoromethyl)-4-pyridinyl]amino}-N-methylbenzamide (Intermediate 1, 60 mg, 0.182 mmol), 4-(4-morpholinyl)aniline (39.0 mg, 0.219 mmol) and 0.4 mL of 1.0 M hydrochloric acid in 2.5 mL of 1,4-dioxane, then irradiated in a microwave oven at 160° C. for 1 hour. The resulting crude material was purified using reverse-phase HPLC to give the title compound (23 mg, 23% yield). MS: M(C23H24F3N5O2)=471.48, (M+H)+=472; 1H NMR (400 MHz, DMSO-d6) δ ppm 2.76 (d, J=4.55 Hz, 3 H) 3.01-3.03 (m, 4 H) 3.72-3.74 (m, 4 H) 6.67 (s, 1 H) 6.88 (d, J=8.84 Hz, 2H) 7.10 (dd, J=15.03, 1.14 Hz, 1H) 7.41 (m, J=9.09 Hz, 2 H) 7.47-7.61 (m, 2 H) 7.70 (dd, J=7.83, 1.52 Hz, 1 H) 8.22 (s, 1 H) 8.67 (d, J=4.55 Hz, 1 H) 9.01 (s, 1 H) 10.15 (s, 1 H).
example 3
[0110]N-Methyl-2-{[2-{[-2-(methyloxy)-4-(4-morpholinyl)phenyl]amino}-5-(trifluoromethyl)-4-pyridinyl]amino}benzamide
[0111]2-{[2-Chloro-5-(trifluoromethyl)-4-pyridinyl]amino}-N-methylbenzamide (Intermediate 1, 50 mg, 0.152 mmol) and [2-(methyloxy)-4-(4-morpholinyl)phenyl]amine (148 mg, 0.607 mmol) were combined with 1M hydrochloric acid (0.303 mL, 0.303 mmol), 1,4-dioxane (0.2 mL) and water (2.5 mL) in a microwave tube, then irradiated in a microwave oven at 170° C. for 25 min. The resulting crude material was filtered and the filtrate was purified by reverse-phase HPLC to give the title compound (35.5 mg, 0.071 mmol, 46.7% yield). MS: M(C25H26F3N5O3)=471.48, (M+H)+=472; 1H NMR (400 MHz, METHANOL-d4) δ ppm 2.88 (s, 3 H), 3.05-3.14 (m, 4 H), 3.78-3.91 (m, 7 H), 6.45 (s, 1 H), 6.52 (dd, J=8.59, 2.53 Hz, 1 H), 6.64 (d, J=2.53 Hz, 1 H), 7.03-7.13 (m, 1 H), 7.30 (d, J=8.59 Hz, 1 H), 7.40-7.50 (m, 2 H), 7.61 (dd, J=7.83, 1.26 Hz, 1 H), 8.10 (s, 1 H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Frequency | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com