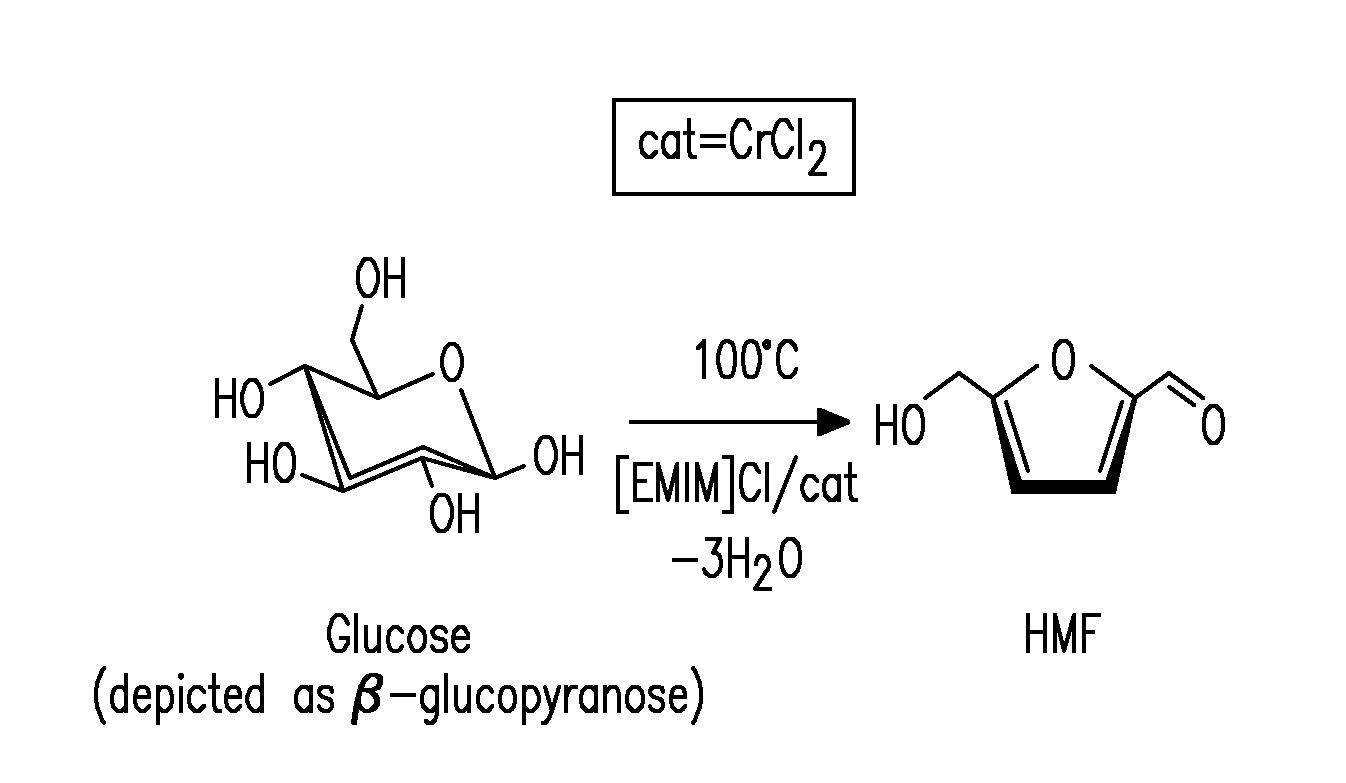
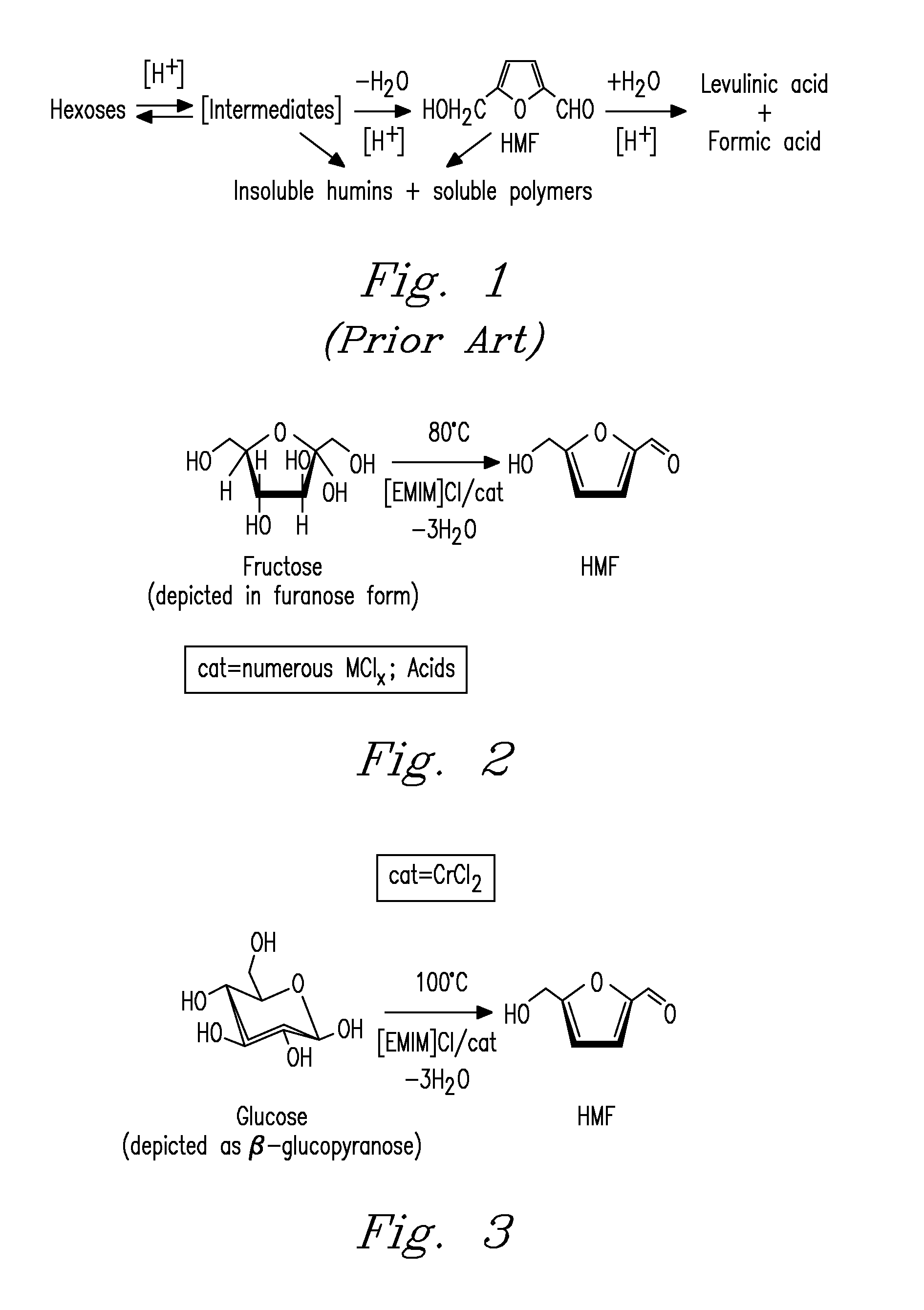
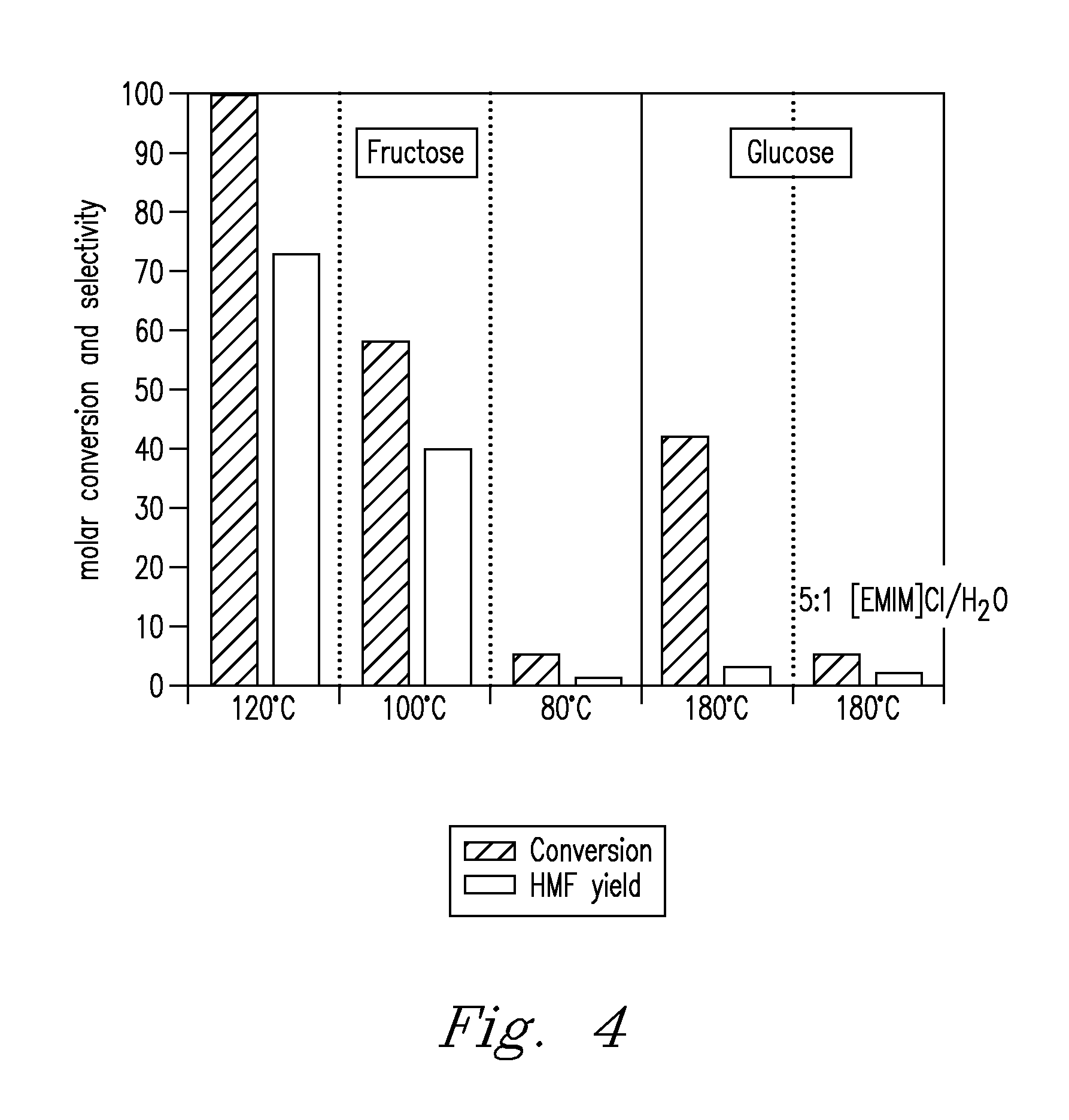
Methods for conversion of carbohydrates in ionic liquids to value-added chemicals
a technology of ionic liquids and conversion methods, applied in the field of conversion methods of carbohydrates in ionic liquids to value-added chemicals, can solve the problems of high production costs, inability to effectively utilize 5-carbon (c5) and 6-carbon (c6) carbohydrate building blocks derived from nature, and limited industrial availability and use of hm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Conversion of Fructose to HMF in [EMIM]Cl
[0084]Fructose (99.9%) was supplied by Mallinckrodt. [EMIM]Cl (99%) was supplied by Solvent-Innovation (GmbH, Cologne, GE). Metal halide catalysts were CuCl, CuCl2, CuBr2, MoCl3, FeCl2, FeCl3, CrCl2, CrCl3, VCl3, AlCl3, MnCl3, PdCl2, PtCl2, PtCl4, RuCl3, RhCl3 were supplied by Sigma-Aldrich (St. Louis, Mo., USA) Acid catalyst was H2SO4, supplied by Sigma-Aldrich (St. Louis, Mo., USA). 500 mg [EMIM]Cl was loaded into reaction vials. Metal halide catalysts were added to respective vials at a concentration of ˜6 mol % with respect to fructose. 2 mg CrCl2 was added to its reaction vial. Vials were installed into the high pressure reactor, heated at 150° C. and shaken at 700 rpm to mix contents. After cooling, 50 mg fructose was added to each vial and heated at 80° C. for 3 h. After cooling, 2.0 mL of water was added for analysis by HPLC. Results are presented in TABLE 1 (see FIG. 5).
TABLE 1Conversion of Fructose to HM...
example 2
Conversion of Fructose to HMF in Alternate Ionic Liquids
Metal Halide or Acid Catalyst
[0085]Fructose was processed as in Example 1 in various ionic liquids containing a metal halide or acid catalyst. Ionic liquids were [EMIM]CH3SO3 (Solvent-Innovations, GmbH, Cologne, GE); tetrabutylammonium chloride (Fluka-Sigma-Aldrich, Steinheim, GE); tetrabutylphosphonium chloride (Ionic Liquid Technologies, GmbH, Denzlingen, GE); 1,2,4-trimethylpyrazolium methyl sulfate (Fluka-Sigma-Aldrich, Steinheim, GE). [EMIM]CH3SO3, tetrabutylphosphonium chloride, and 1,2,4-trimethylpyrazolium methyl sulfate each contained a catalytic quantity of acid. Results are presented in TABLE 2.
TABLE 2Conversion of Fructose to HMF, and product yields.FeedstockconversionProductExampleFeedstockIonic Liquid (IL) and Catalyst(%)Yields (%)2.1FructoseIL: [EMIM]CH3SO3;99.6HMF: 86.5Catalyst: acid2.2FructoseIL: tetrabutylammonium chloride;—HMF: 59.1Catalyst: VCl32.3FructoseIL: tetrabutylphosphonium chloride;—HMF: 65.2Catalyst...
example 3
Carbohydrate Reactivity in “as-Received” and Purified Ionic Liquid
[0086]Carbohydrate reactivity was compared in both “as-received” (as purchased) and purified ionic liquid. Fructose was processed as in Example 1 in 99% [EMIM]CH3SO3 (Solvent-Innovation, GmbH, Cologne, GE) in both the “as-received” ionic liquid and the ionic liquid purified with basic alumina to remove any contaminants (e.g., methane sulfonic acid). Reaction time and temperature was 3 h at 80° C. Conversion of fructose in the “as-received” ionic liquid was 99.9%; yield of HMF was 83.9%. Conversion of fructose in purified ionic liquid was 0%; yield of HMF was 0%. Results demonstrate that some impurities present in ionic liquids (e.g., as purchased) are sufficient to catalyze reaction of carbohydrates. When purified, the ionic liquid does not exhibit reactivity at the same temperature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction time | aaaaa | aaaaa |
| reaction time | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com