Ex Vivo Remodeling of Excised Blood Vessels for Vascular Grafts
a technology of excised blood vessels and vascular grafts, applied in blood vessels, medical science, prosthesis, etc., can solve problems such as hampered identification of specific mechanical stimuli responsible for remodeling specific aspects, and achieve the effect of increasing the diameter, length, and wall thickness of the excised vessel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Perfusion System: Control of Mechanical Environment
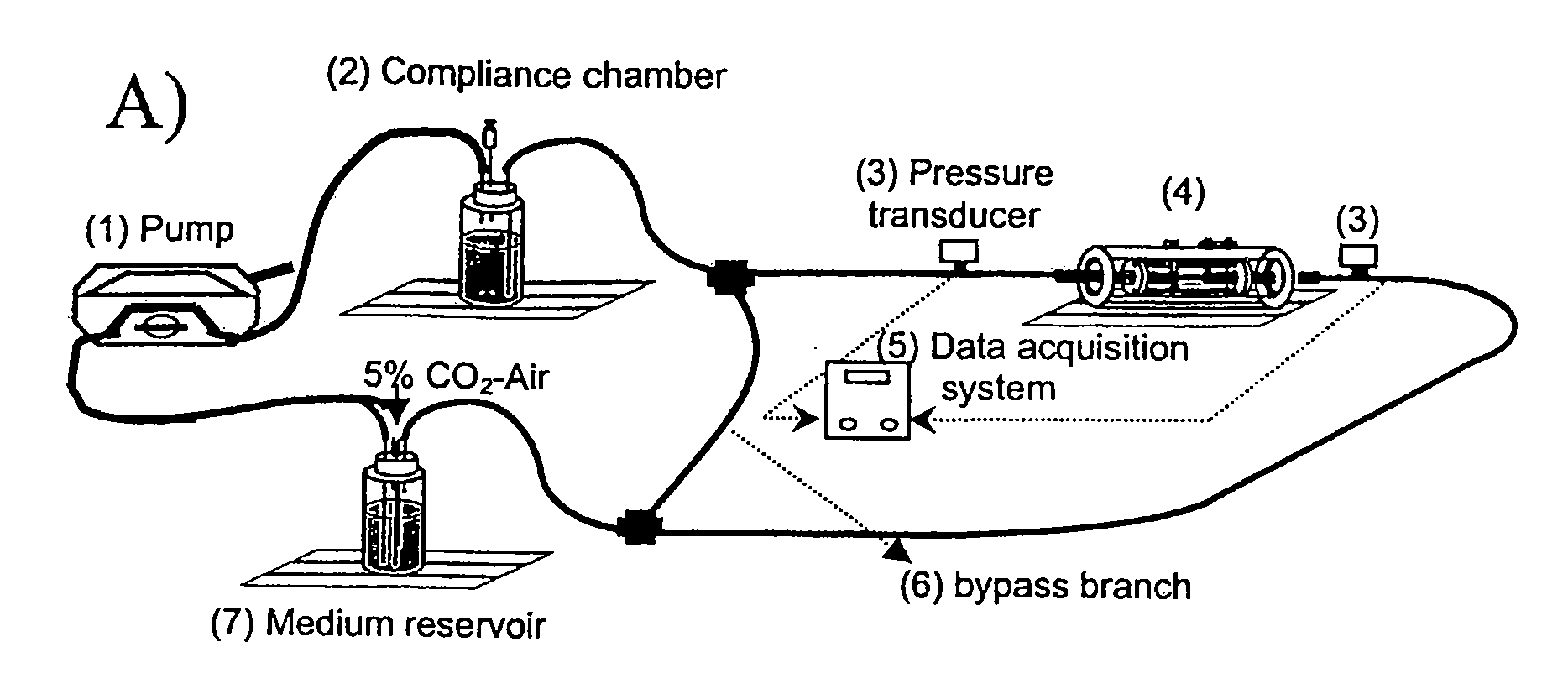
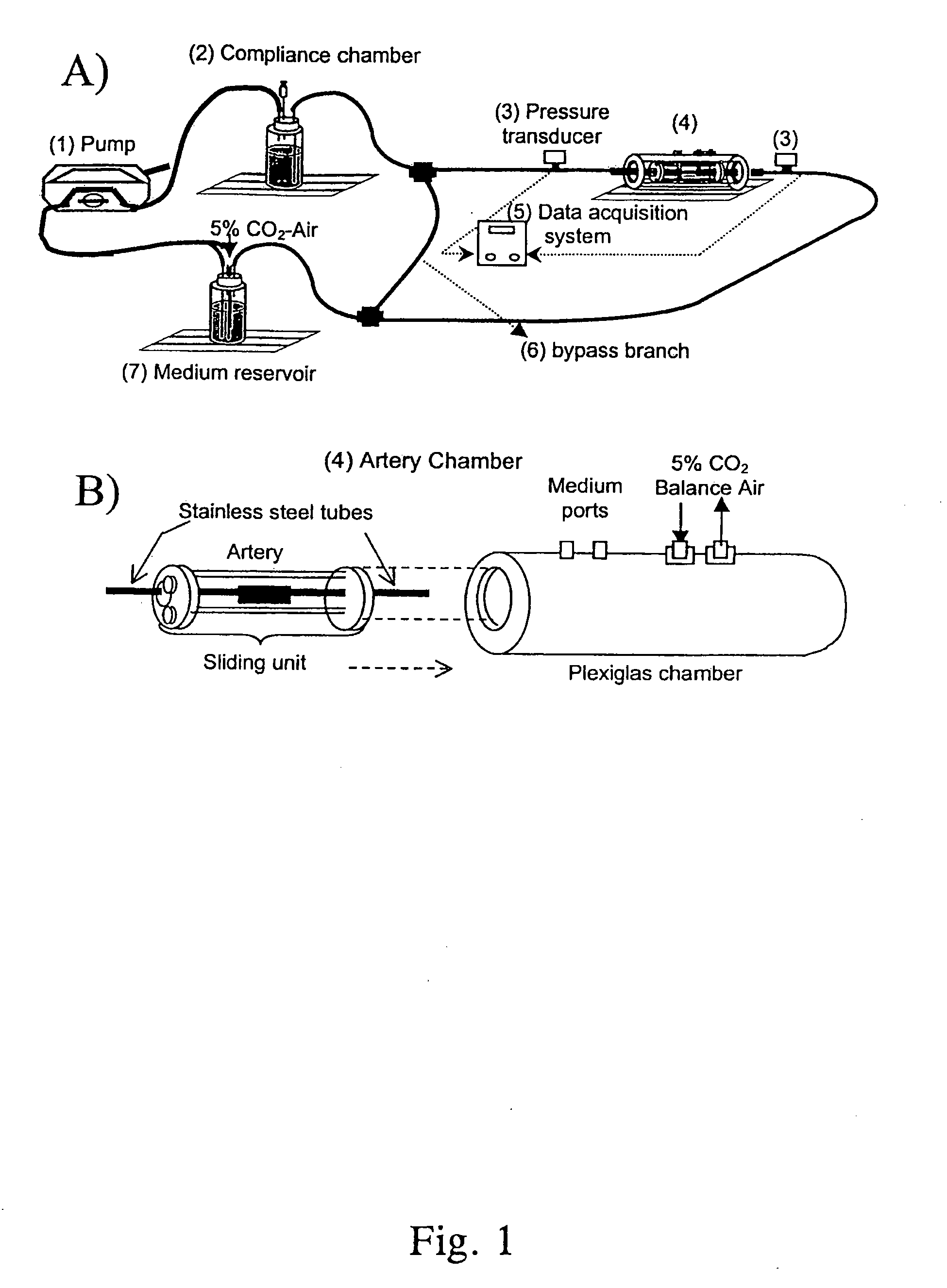
[0080]The perfusion system consisted of a peristaltic pump, compliance chamber, artery chamber, and reservoir, all connected using Tygon laboratory tubing (Formula R-3603, Fisher Scientific, Pittsburgh, Pa.), ports for injection into or sampling from the perfusing medium, and pressure transducers (Model MER100, Triton Technology, Inc., San Diego, Calif.) upstream and downstream from the artery (FIGS. 1A and 1B). Steady flow was provided by a Masterflex roller pump (1) (Model 7553-70, Cole-Parmer, Vernon Hills, Ill.) with Masterflex Tygon LFL pump tubing (Formula 06429-25, Fisher Scientific, Pittsburgh, Pa.). Real-time pressure data were acquired via an analog-digital board (Model PCI-6023E, National Instruments, Austin, Tex.) connected to a Triton System 6 Twinpak Chassis (Active Redirection Transit-Time Flow Module, Model 200-206 and Dual Pressure Amplifier Module, Model 200-204, Triton, San Diego, Calif.). Data were visualized...
example 2
Determining which Mechanical Factors Regulate Remodeling of Arteries
[0084]Artery Harvest, Preparation and Maintenance: Carotid arteries from neonatal (˜5-kg) and juvenile (˜30-kg) pigs were harvested by cardiothoracic surgeons at the Children's Hospital of Philadelphia after the animals were euthanized. Carotid arteries from adult pigs (˜100-kg) were obtained from freshly exsanguinated pigs at a local abattoir. Arteries were transported in ice-cold culture medium (Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U / mL penicillin and 100 μg / mL streptomycin, all from Life Technologies, Inc., Rockville, Md.). Upon arrival, arteries were prepared within a laminar flow hood using sterile instruments.
[0085]Arteries, measuring 3-6 cm in length, were individually cleaned of excess adventitial and connective tissue. Sections were taken for histology, methylthiazol tetrazolium (MTT) assay and, in some cases, dry weight and / or mechanical testing. Dry weigh...
example 3
Control of Extravascular Pressure
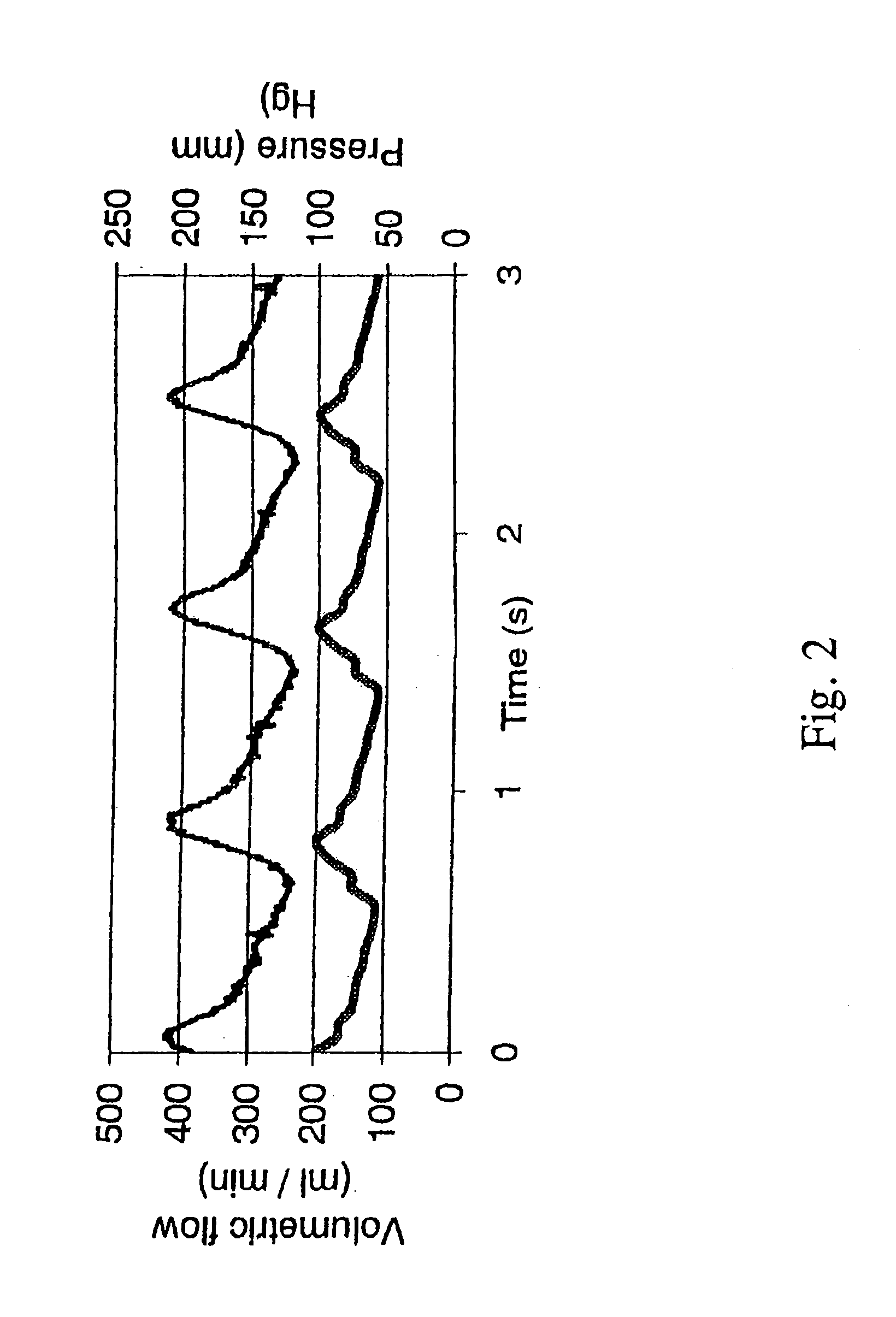
[0116]Since vessels are compliant viscoelastic materials, adequate control of the extravascular pressure was essential to validate the accuracy of the estimates for transmural pressure. Accordingly, to critically control extravascular pressure (i.e., the pressure of the medium bathing the external surface of the vessel), pressures were measured by placing a catheter pressure transducer close to the external surface of a segment of compliant Penrose tubing used as a surrogate of an artery for these preliminary studies and compared measured values to the set point values.
[0117]A goal of this experiment was to reduce both the magnitude and the variation in the transmural pressure across a compliant tube (e.g., 30±10 mm Hg controlled, as opposed to 120±25 mm Hg uncontrolled). As shown in FIG. 3B, these data indicate that extravascular pressure was accurately controlled to a constant value, and transmural pressure was controlled with a degree of success w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com