Compositions, devices, systems, and methods for using a nanopore
a nanopore and nanopore technology, applied in the field of nanopore analysis techniques, can solve the problems of difficult correlation between the length of any given polynucleotide and its translocation time, and the control of the rate at which the target polynucleotide is analyzed in the nanopore analysis technique, so as to maintain enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0248]Herein are described several examples to demonstrate the capability of measuring macromolecules and polanions or polycations.
example i
Enzyme Binding is Prevented by a Blocking Primer
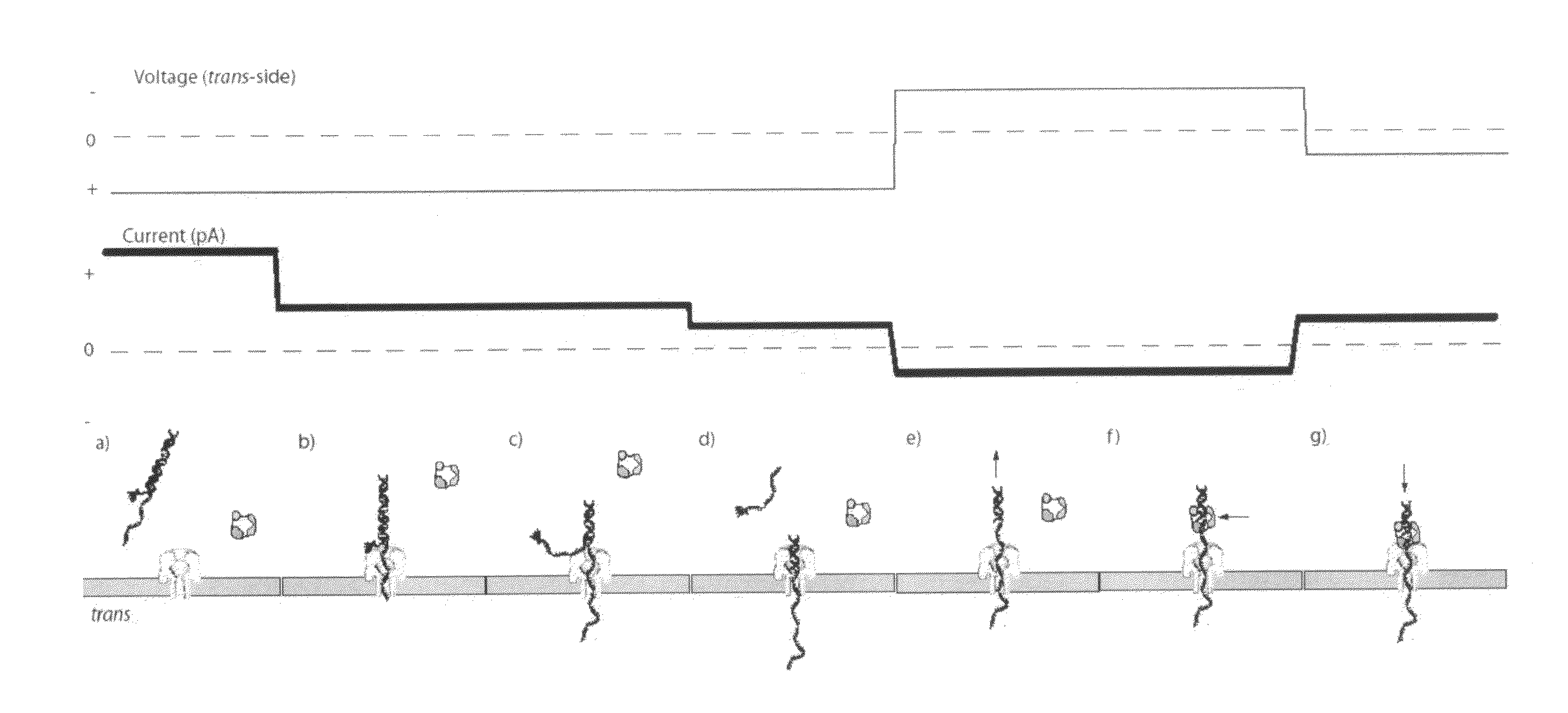
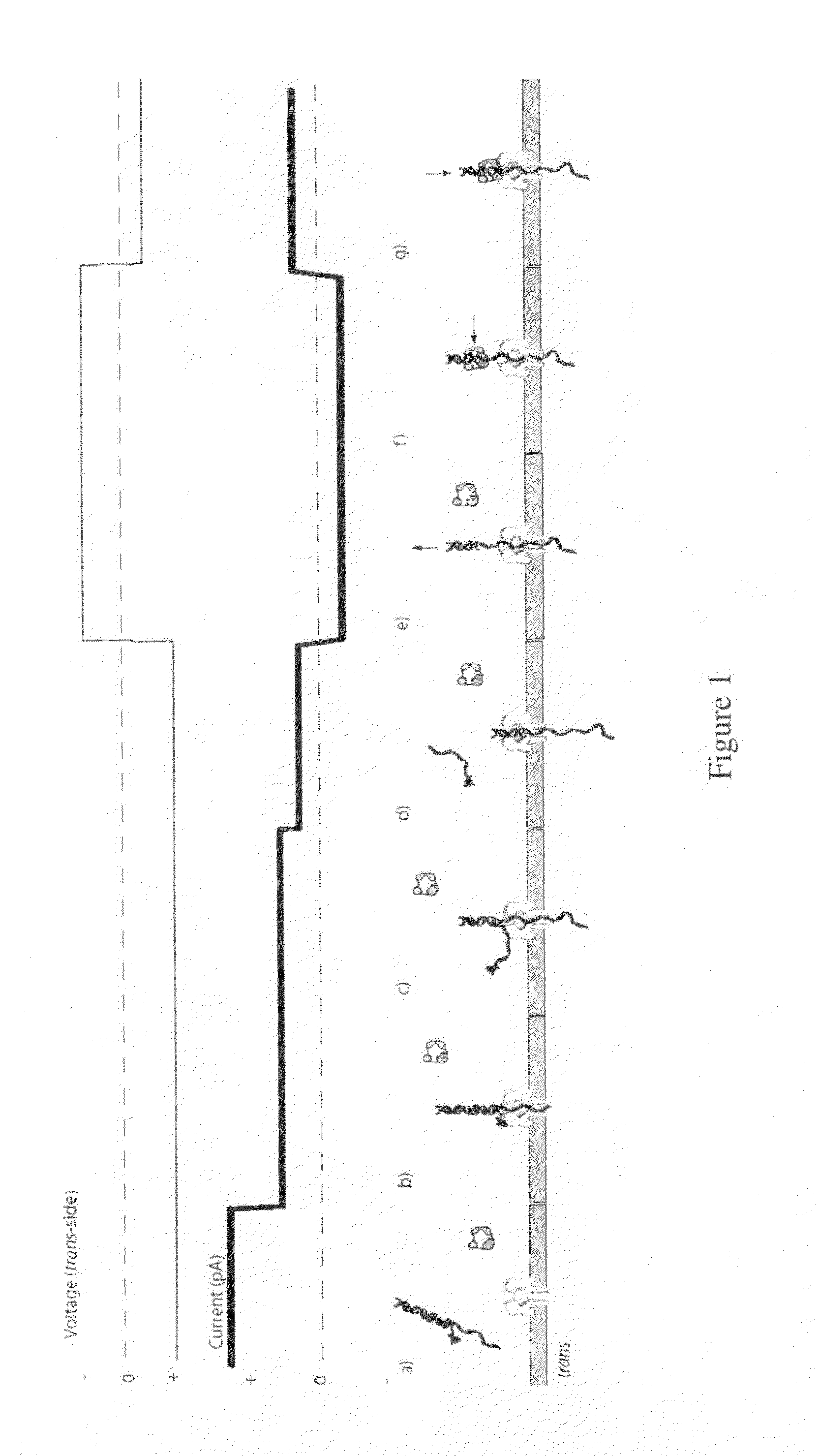
[0249]For an illustration of this method, see FIGS. 1(a) through 1(g). (a) In this scenario, the blocking primer is bound to the primer / template in bulk phase. Structure of the ternary complex prevents binding of the enzyme to the junction between the dsDNA and ssDNA segments of the target DNA where the first nucleotide would be incorporated. (b) Capture of a blocked primer / template under an applied voltage (trans side positive) threads the ssDNA into the pore and perches the dsDNA above the vestibule. This occurs because the loop at the end of the blocking primer is too large to enter the vestibule. The current reports capture of the complex in this state. (c) Under the applied voltage, the ssDNA segment advances in the pore toward the trans-side and processively unzips base-pairs between the blocking primer and the template. The energy cost of releasing each base pair independently is small (about 2.5 kcal / mol), so it proceeds rapidl...
example ii
Enzyme Catalysis is Prevented by a Blocking Primer
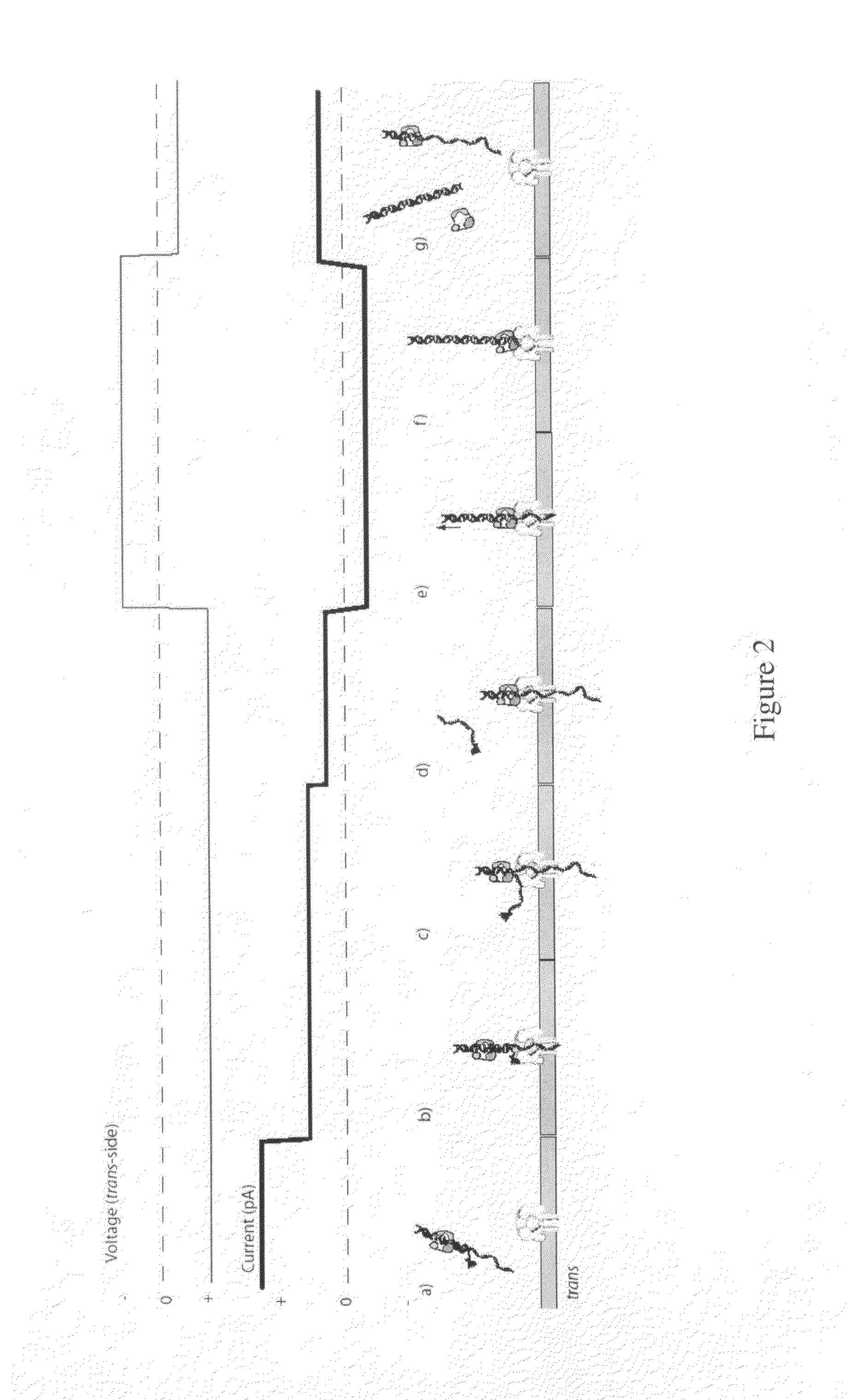
[0250]For an illustration of this method, see FIGS. 2(a) through 2(g). (a) In this scenario, the blocking primer is bound to the primer / template in bulk phase. Structure of the ternary complex permits binding of the enzyme to the target DNA but catalysis and processing along the template are prevented. (b) Capture of a blocked primer / template under an applied voltage (trans-side positive) threads the ssDNA into the pore and perches the dsDNA above the vestibule. This occurs because the loop at the end of the blocking primer is too large to enter the vestibule. The current reports capture of the complex in this state. (c) Under the applied voltage, the ssDNA segment advances in the pore toward the trans-side and processively unzips base-pairs between the blocking primer and the template. The energy cost of releasing each base pair independently is small (about 2.5 kcal / mol), so it proceeds rapidly under force. During this unzipping pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com