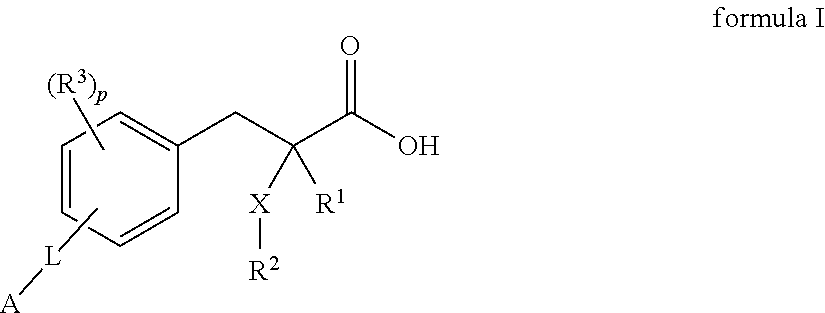
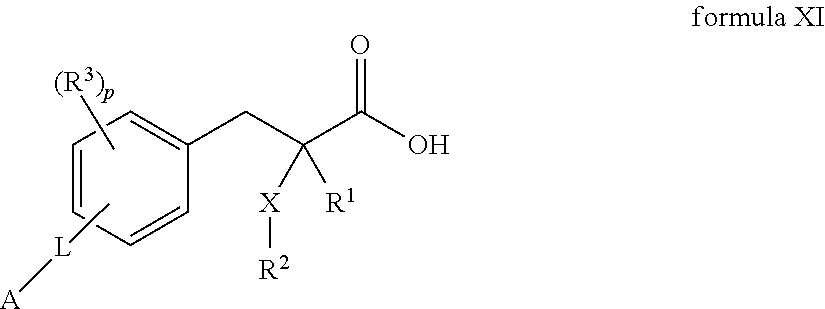
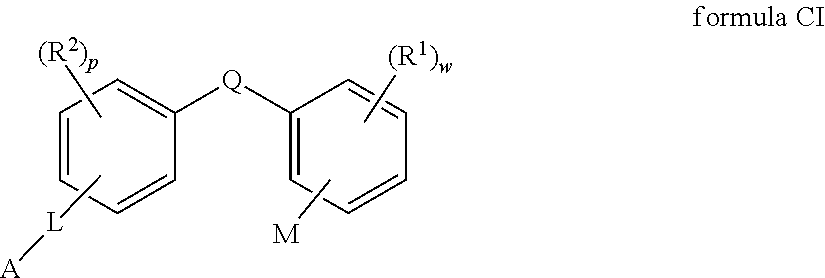
2 -substituted- 3 -phenylpropionic acid derivatives and their use in the treatment of inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-Methanesulfonyloxy-benzoic acid 4-{2-carboxy-2-[2-(4-fluoro-phenyl)-ethylsulfanyl]-ethyl}-benzyl ester
[0531]
[0532]Step 1: To a solution of 2-[2-(4-fluoro-phenyl)-ethylsulfanyl]-3-(4-hydroxymethyl-phenyl)-propionic acid 2,2,2 trichloro-ethyl ester (70 mg, 0.15 mmol) and (4-methanesulfonyloxy-phenyl)-acetic acid (32.5 mg, 0.15 mmol) in anhydrous DCM (3.0 mL) was added EDCxHCl (37.5 mg, 0.195 mmol) and DMAP (0.18 mg, 0.002 mmol) under nitrogen atmosphere at 0° C. After complete addition the cooling bath was removed and the homogenous mixture was stirred at ambient temperature until no starting material was left. The mixture was diluted with water and 2N aq. HCl and extracted with DCM. The combined organic layers were dried (MgSO4) and the solvent subsequently evaporated under reduced pressure to afford the desired ester intermediate which was used in the next step without further purification.
[0533]Step 2: The crude 2,2,2-trichloro ethyl ester derivative (68.7 mg, 0.10 mmol) was diss...
example 2
2-[2-(4-Fluoro-phenyl)-ethylsulfanyl]-3-{4-[2-(4-methanesulfonyloxy-phenyl)-acetoxymethyl]-phenyl}-propionic acid
[0534]
[0535]The title compound was prepared from 2-[2-(4-fluoro-phenyl)-ethylsulfanyl]-3-(4-hydroxymethyl-phenyl)-propionic acid 2,2,2 trichloro-ethyl ester (33 mg, 0.072 mmol) and (4-methanesulfonyloxy-phenyl)-acetic acid (15 mg, 0.065 mmol) in the same manner as described for example 1. The crude product was purified by flash chromatography (yield: 2.0 mg, 5.6%). Mass Spectrum: M+H+547.10.
example 3
2-[2-(4-Fluoro-phenyl)-ethylsulfanyl]-3-[4-(4-trifluoromethyl-benzyloxycarbonylmethyl)-phenyl]-propionic acid
[0536]
[0537]The title compound was prepared from 3-(4-carboxymethyl-phenyl)-2-[2-(4-fluoro-phenyl)-ethylsulfanyl]-propionic acid 2,2,2-trichloro ethyl ester (30 mg, 0.061 mmol) and 4-trifluoromethylbenzyl alcohol (12 mg, 0.067 mmol) in the same manner as described for example 1. The crude product was purified by HPLC (yield: 3.0 mg, 9.4%). 1H NMR (400 MHz, CDCl3): δ 7.56 (d, 2H), 7.36 (d, 2H), 7.08-7.19 (m, 6H), 6.90-7.04 (m, 2H), 5.14 (s, 2H), 3.63 (s, 2H), 3.45 (dd, 2H), 3.15 (dd, 2H), 2.80-2.92 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com