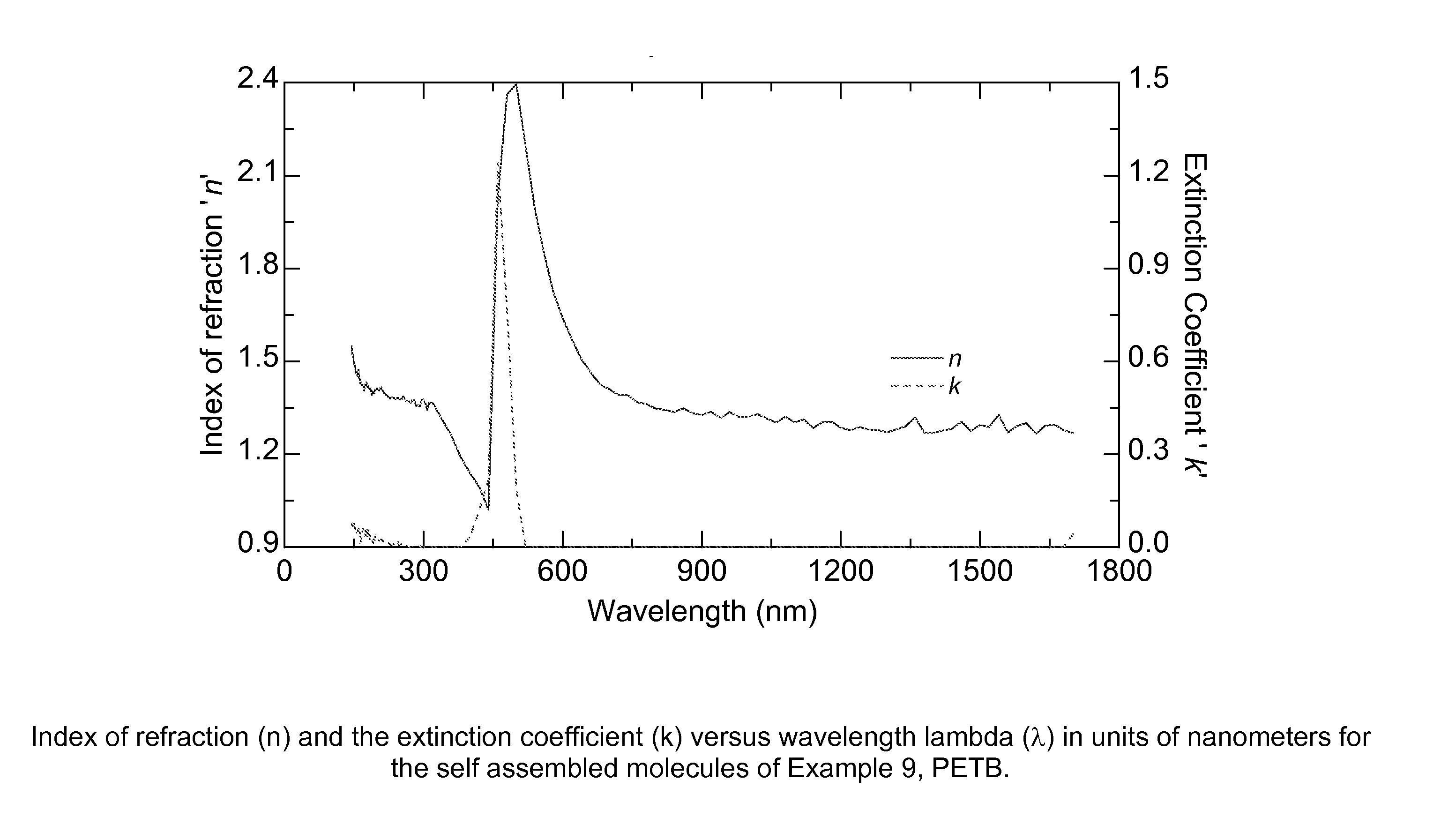
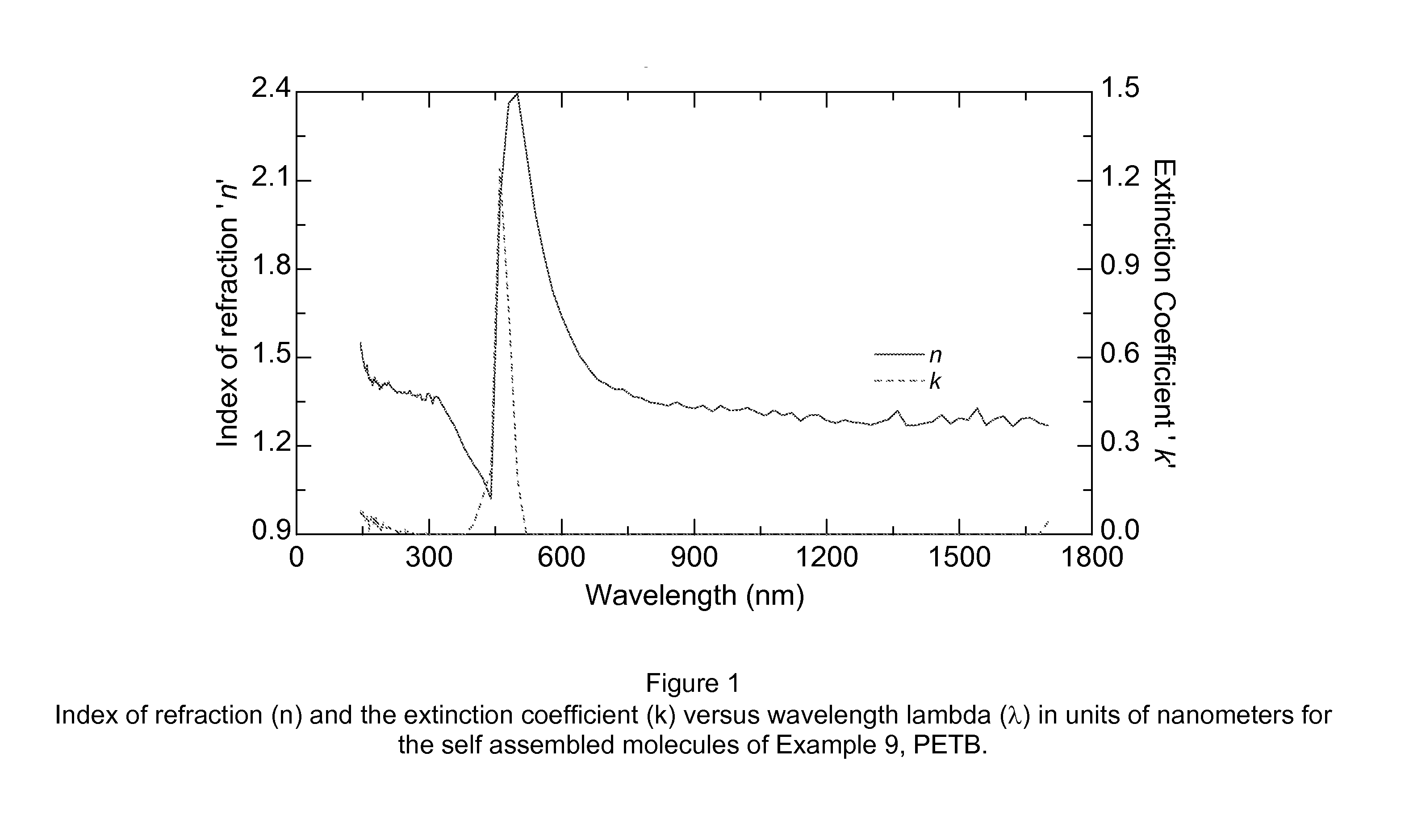
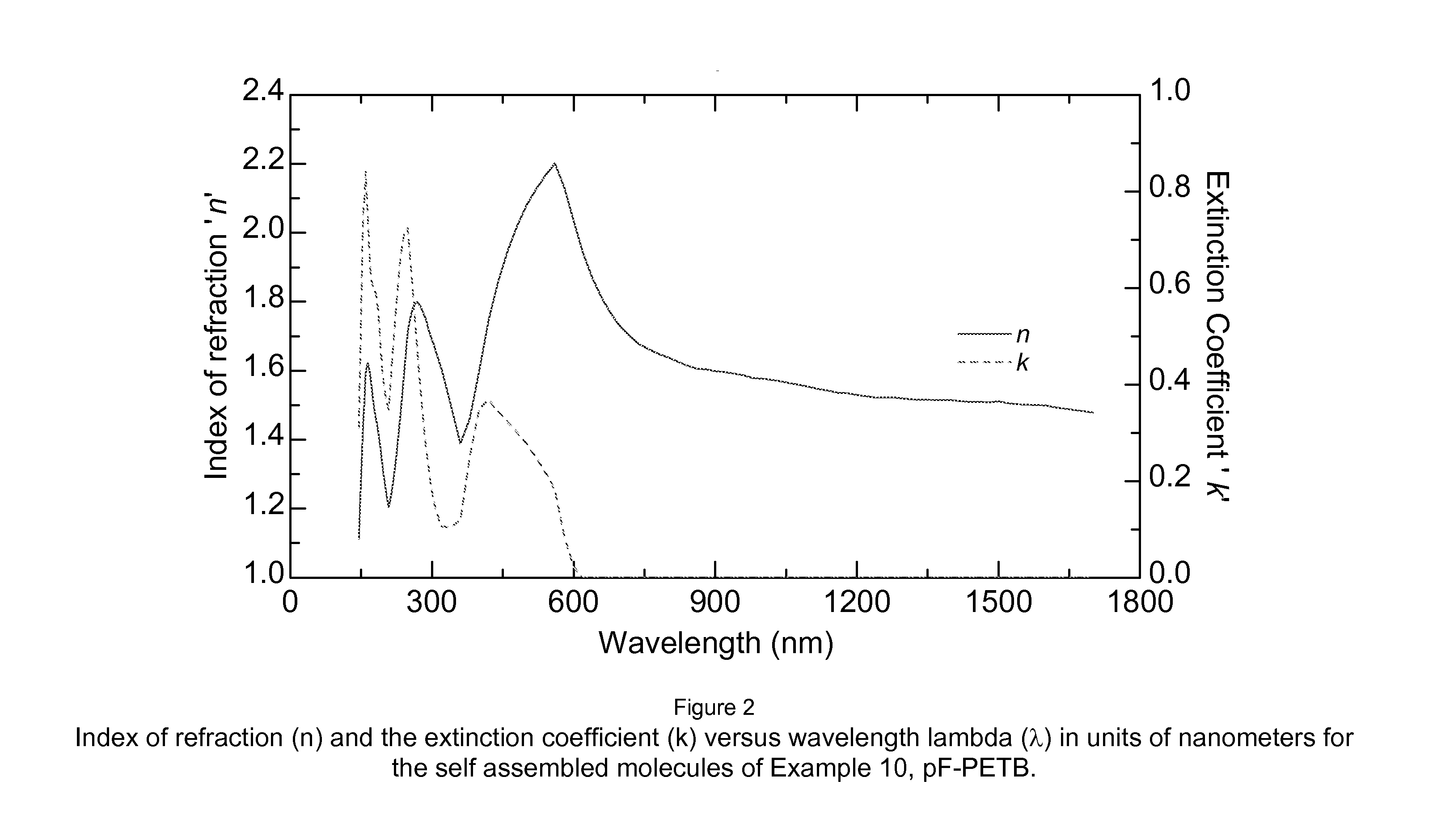
Aromatic and aromatic/heteroaromatic molecular structures with controllable electron conducting properties
a molecular structure and electron conducting technology, applied in the direction of non-metal conductors, conductors, transportation and packaging, etc., can solve the problems of difficult connection of conductive molecules to electrodes, difficult conductivity measurement of single molecules, and difficulty in discovering new conductive molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1,4-Bis(4-Pyridyl)Butadiyne)
Reagents Used Include:
[0055]4-Ethynylpyridine hydrochloride: 97%, Mw=139.58, Mp 150° C., Aldrich: 53,092-1[0056]Diethyl Ether: Mw=74.12 d=0.713, Bp 35-36° C.[0057]Triethylamine: Mw=101.19 d=0.726, Mp −115° C., Bp 88.8° C., Aldrich: 47,128-3[0058]Copper (I) Chloride: Mw −98.99, Mp 430° C., Aldrich: 22, 962-8[0059]Oxygen: Mw=32, Mp −218° C., Bp −183° C., Aldrich: 29,560-4[0060]4-Ethynylpyridine: Mw=103.1237, Mp 95-96° C.,[0061]Pyridine: Mw=79.10, D=0.983, Bp 115-115° C. Aldrich: 27,097-0
[0062]This compound was synthesized by oxidative coupling of 4-ethynyl pyridine (freshly prepared from its hydrochloride form as described below) in pyridine in the presence of copper (I) chloride according to the following reaction scheme:
[0063]Crystallization from carbon tetrachloride gave colorless plates with the following structure as determined from X-ray diffraction data.
[0064]Crystal Data: C14H8N2, from carbon tetrachloride, colorless, irregular plate,...
example 2
Preparation of 4-Ethynyl(Pyridine)-4′-Ethynylphenyl-5′-Nitro-1-Pyridine
[0068]
This compound was synthesized as described above in Example 1. Crystallization from ethanol gave colorless needles with the following structure as determined from X-ray diffraction data. It can be seen that the mean plane of the outer rings forms an angle of 28.3 degrees with the inner ring.
[0069]Crystal Data: C20H11N3O2, from ethanol, colorless, needle, ˜0.220×0.020×0.020 mm, monoclinic, C2 / c, a=16.351(3) Å, b=11.659(3) Å, c=9.2685(19) Å, beta=117.706(5)°, Vol.=1564.3(6) Å3, Z=4, T=−100° C., Formula weight=325.32, Density=1.381 mg / m3, μ(Mo)=0.09 mm−1.
example 3
Preparation of 4-Ethynyl(Pyridine)-4′-EthynylBiphenylene-1-Pyridine
[0070]
[0071]4-Ethynyl(pyridine)-4′-ethynylbiphenylene-1-pyridine can be synthesized according to the procedure outlined in Example 1 via the reaction scheme shown above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com