Apparatus and method of placement of a graft or graft system
a technology of vascular prosthesis and applicator, which is applied in the field of endoluminal vascular prosthesis, can solve the problems of high mortality, abdominal wall surgery, and sac rupture, and achieve the effect of reducing the risk of surgery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
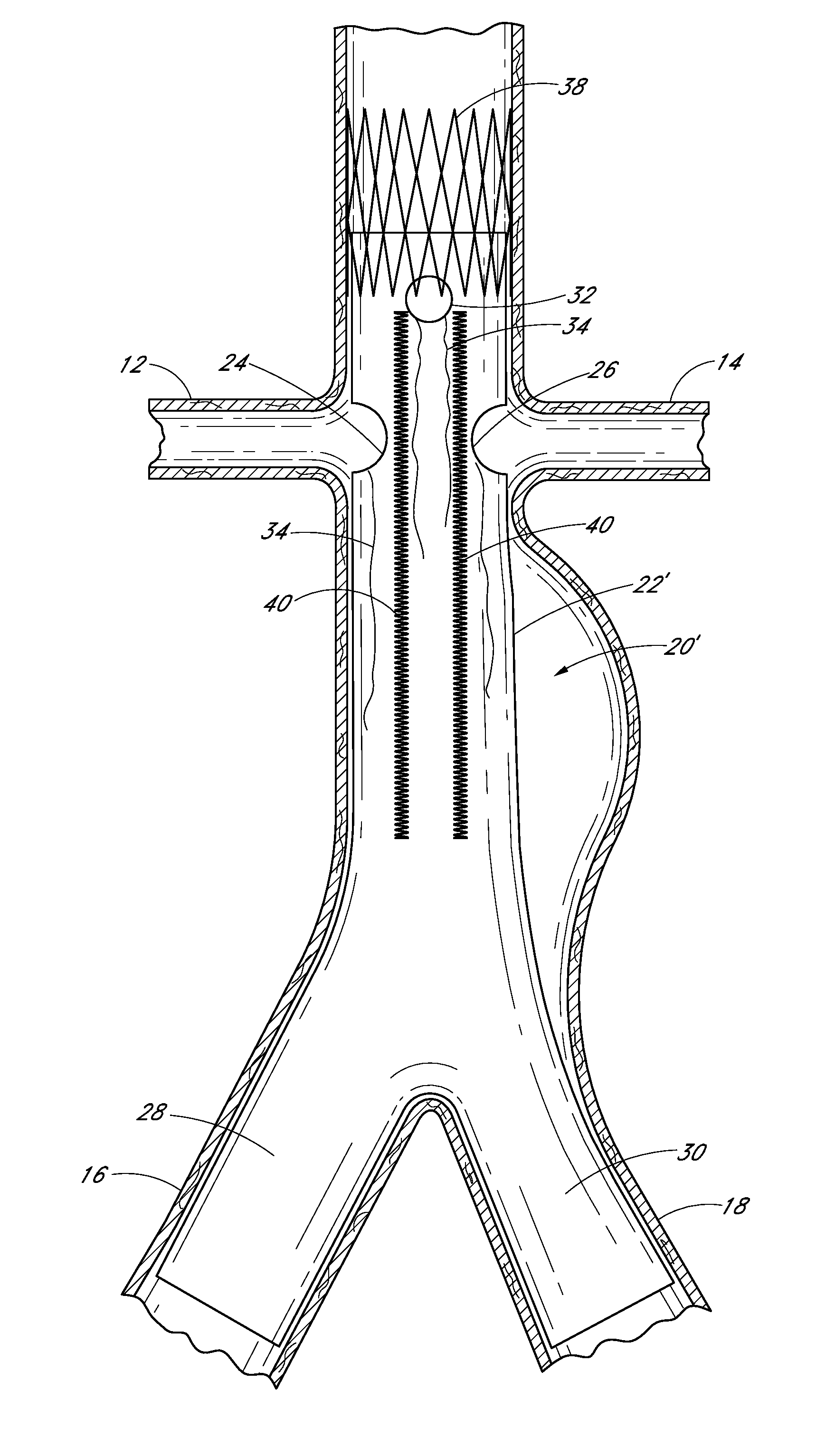
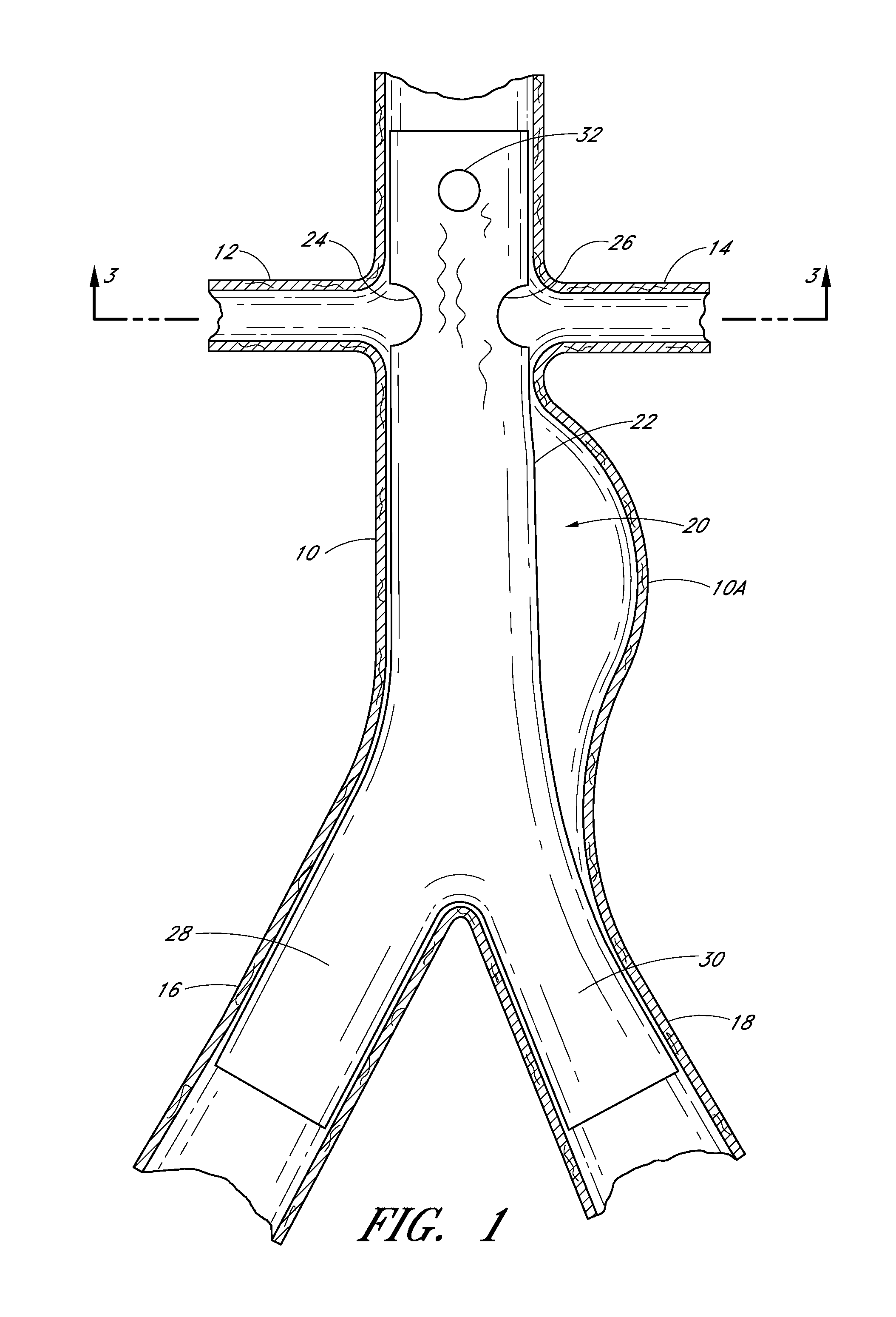
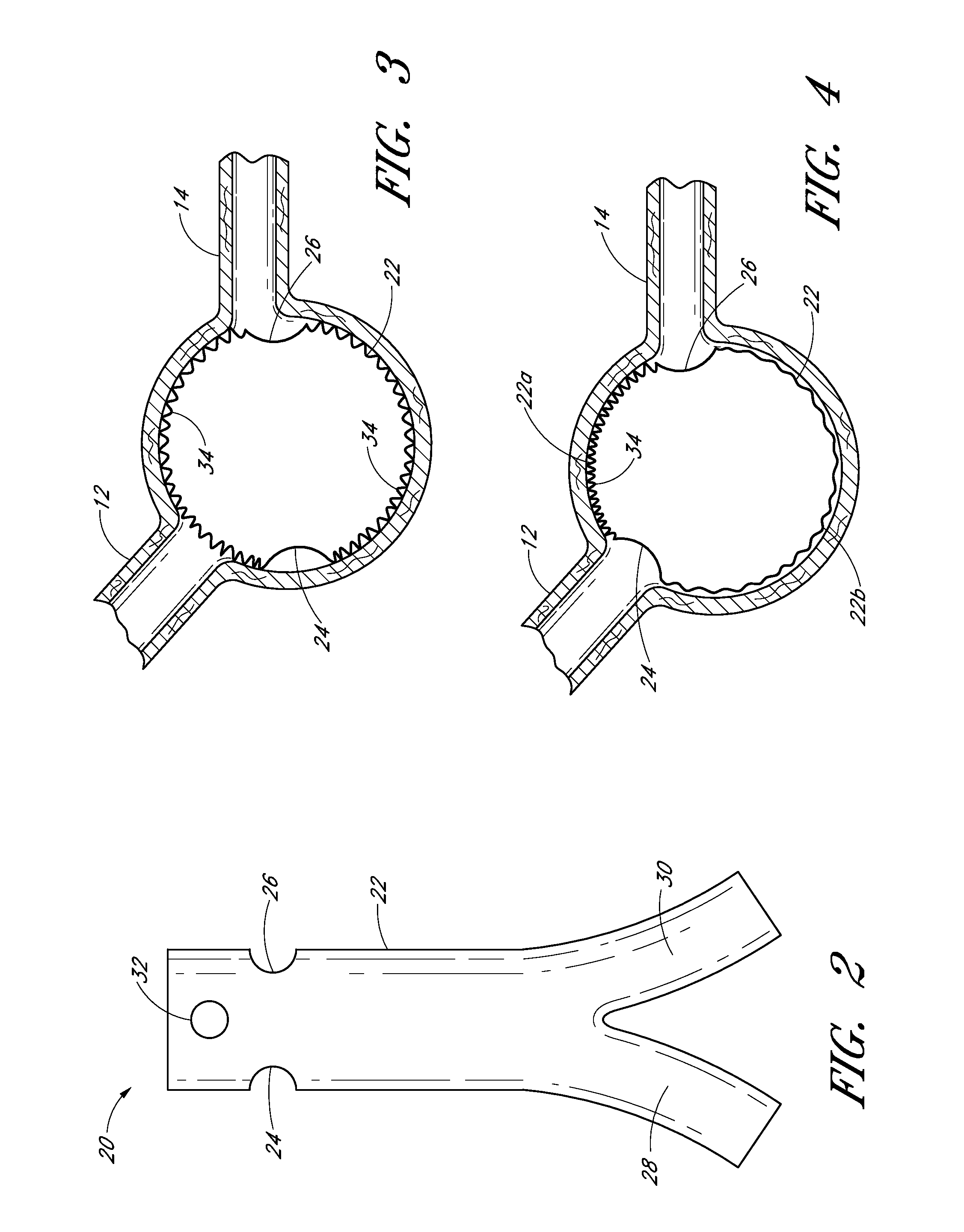
[0016]Some embodiments of the endoluminal prostheses disclosed (directly and / or by incorporation by reference) herein pertain to designs and methods of placement of a branch graft or branch graft system having lateral openings in the main graft. The main graft can be positioned within the main blood vessel such as the aorta so that the lateral openings (also referred to herein as fenestrations) can be aligned with the branch blood vessels, to allow blood to flow through the openings in the main graft and into the branch vessels. Because the axial and angular position of the branch blood vessels can vary from one patient's anatomy to the next, the embodiments of the graft systems disclosed herein can allow a surgeon to adjust the position of the fenestrations so as to align the fenestrations with the branch vessels so that blood flow through the branch vessels is not obstructed by the main graft.
[0017]The branch graft system can comprise a tubular expandable main body and at least on...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com