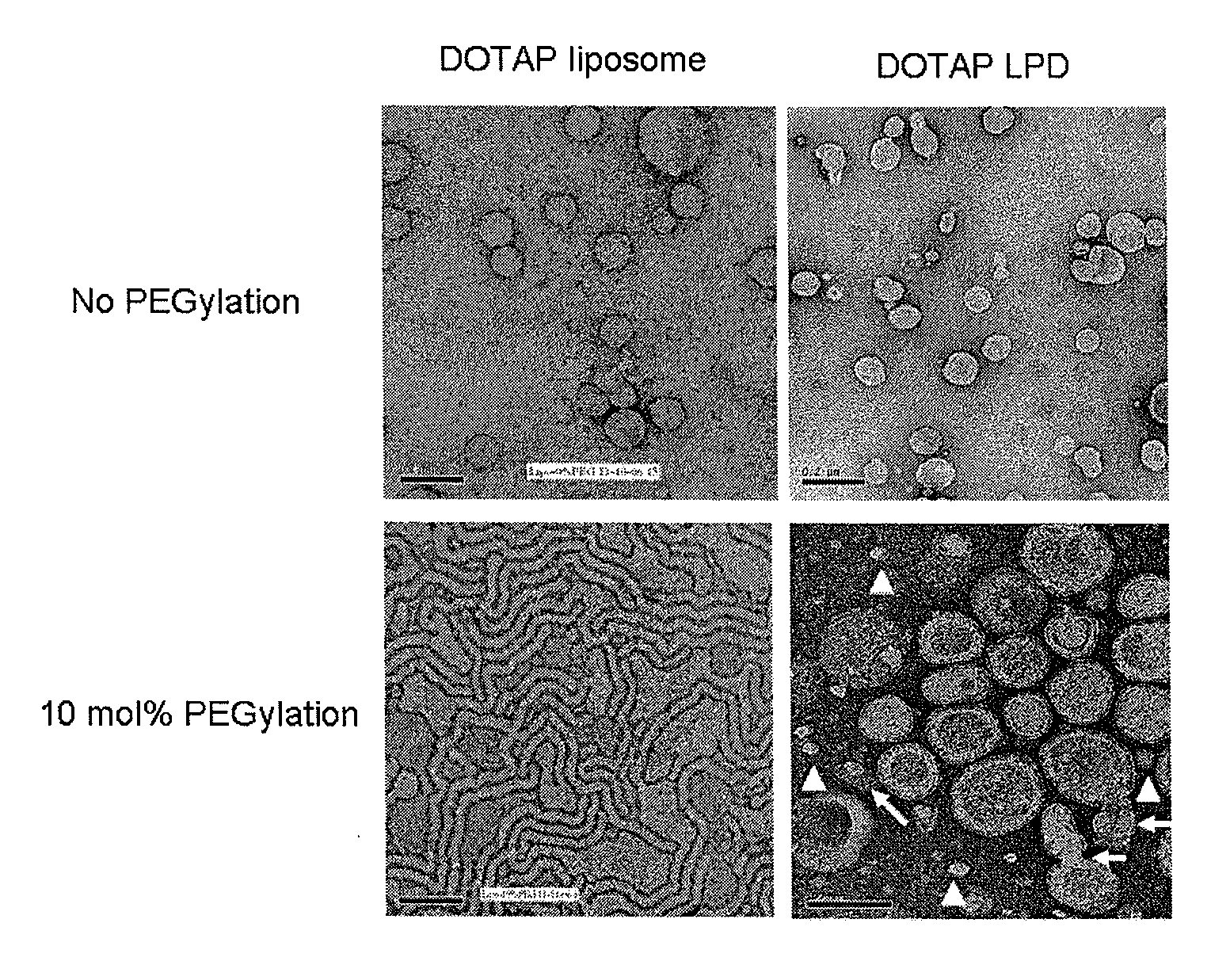
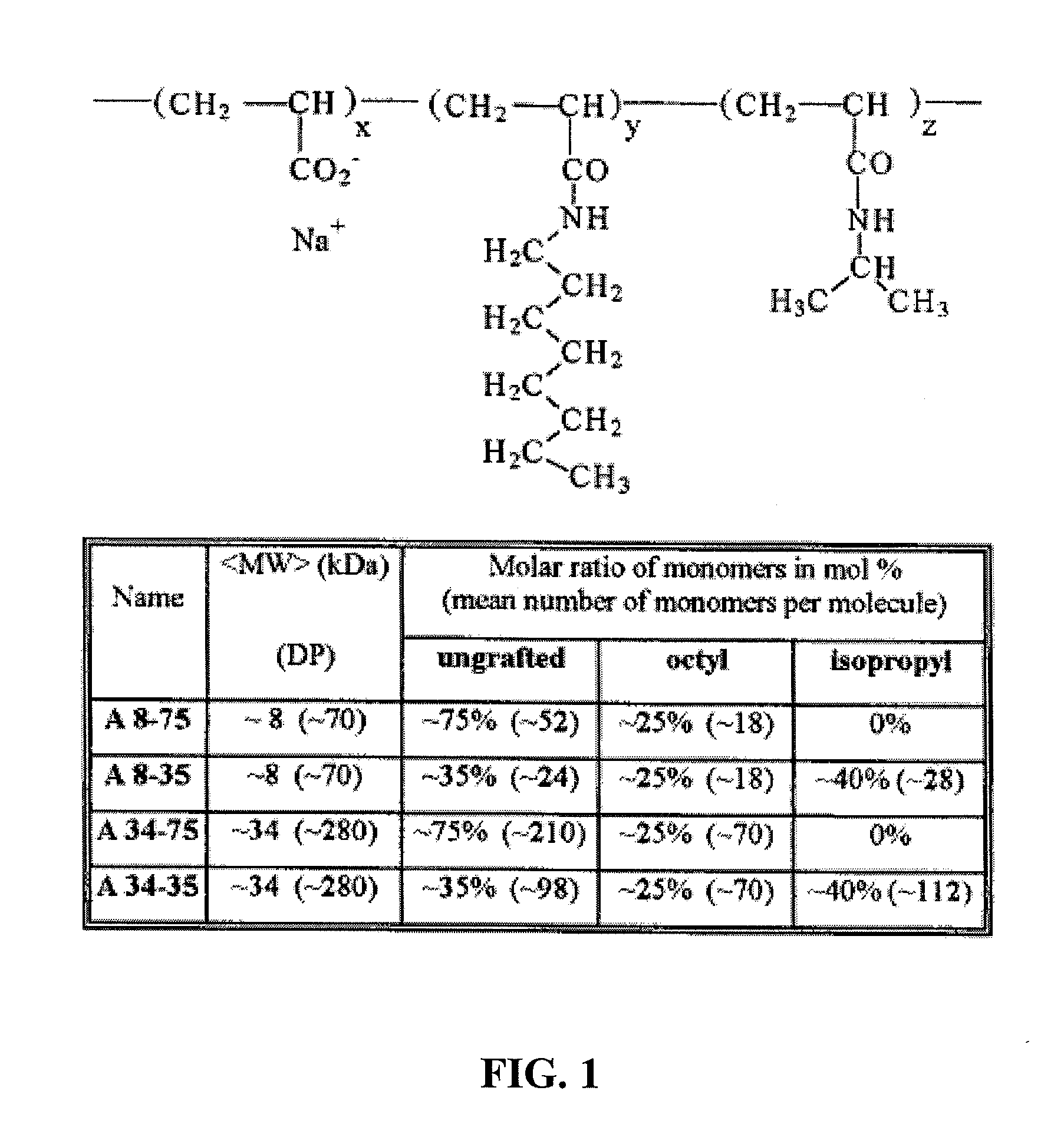
Methods and compositions for the delivery of bioactive compounds
a bioactive compound and composition technology, applied in the direction of drug compositions, tissue culture, dna/rna fragmentation, etc., can solve the problems of undesirable pharmacokinetic properties and toxic side effects, and achieve the effect of reducing the immunogenic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of the LPH-NP Formulation
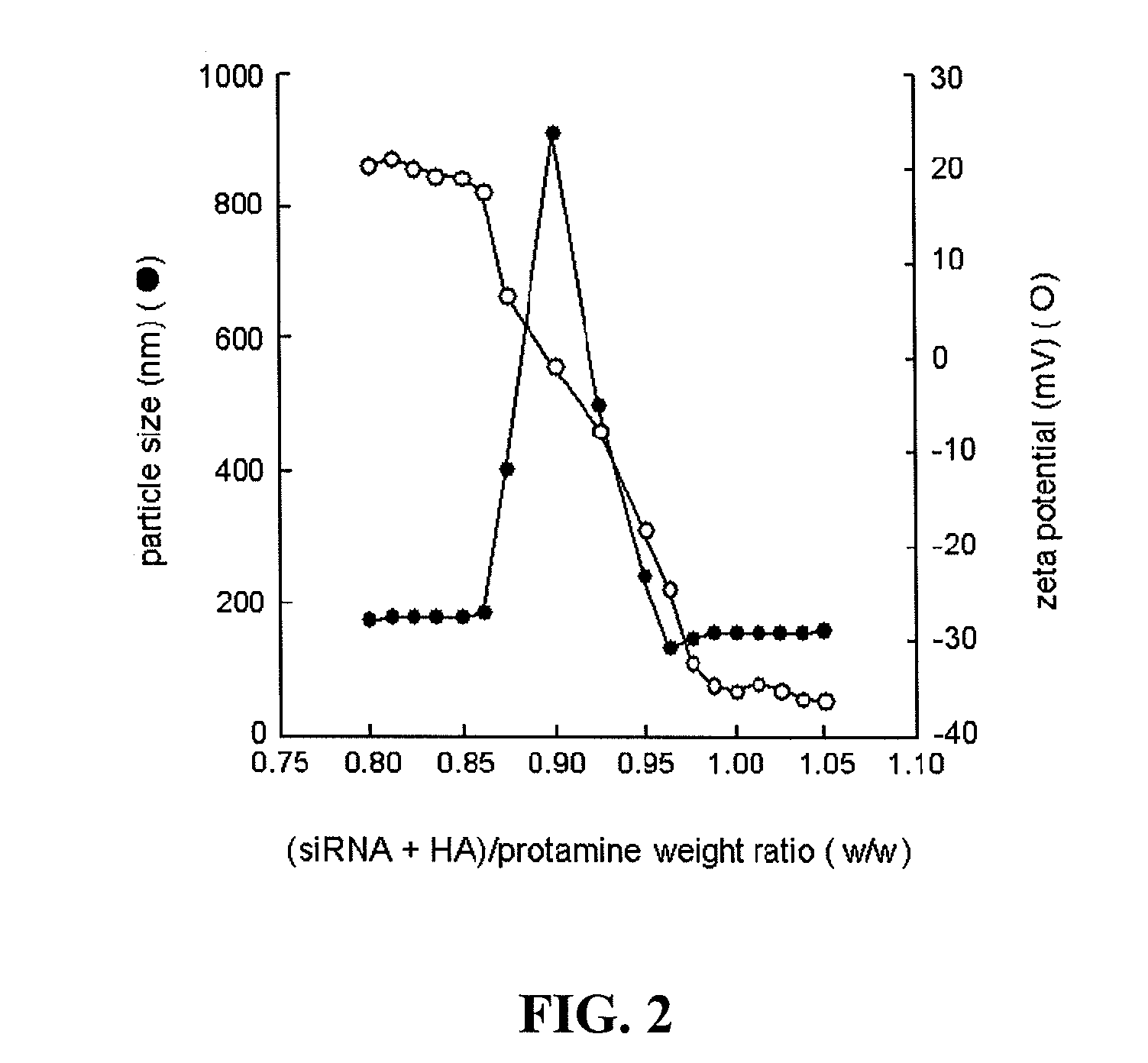
[0342]We prepared the complex of siRNA / hyaluronic acid (HA) and protamine in different ratios and measured their particle size and zeta potential. As shown in FIG. 2, particle size and zeta potential changed according to the ratio of siRNA / HA to protamine. Large aggregates were found at the approximate ratio of 0.9 (siRNA / HA:protamine, weight ratio). At this ratio, a neutral complex was formed (zeta potential ˜0 mV), which tended to aggregate. An increase in the amount of protamine in the complex resulted in an increase in the zeta potential with a dramatic change between ratios 0.865-0.9625 (from −35 mV to 20 mV). We chose 1.0 as the optimal ratio, as the complex stayed negatively charged and had a relatively small size (˜150 nm).
[0343]Next, we mixed the siRNA / HA / protamine complex with different amounts of cationic lipid to prepare the naked LPH-NP and analyzed the size and zeta potential of the resulting particle. As mentioned earlier, a slight...
example 2
In Vitro Intracellular siRNA Delivery and Gene Silencing with Different Formulations
[0346]In vitro intracellular siRNA delivery studies were performed in B16F10 cells (FIG. 6). The fluorescence intensity of cells treated with free siRNA was indistinguishable from background fluorescence levels. This indicates that free siRNA inefficiently penetrates the cell membrane due to its highly hydrophilic nucleic acid backbone. The siRNA delivery efficiency of the targeted LPH-NP (PEGylated with ligand) was significantly higher than that of the non-targeted LPH-NP (PEGylated without ligand), and was inhibited by co-incubation with haloperidol, a known ligand for sigma receptor. This indicates that targeted LPH-NP can deliver siRNA to the B16F10 cells through sigma receptor mediated endocytosis, similar to the targeted LPD-NP (Li et al. (2008) J. Control. Rel. 126:77-84). Interestingly, the delivery efficiency of targeted LPH-NP was significantly higher than that of the targeted LPD-NP. The r...
example 3
In Vivo Luciferase Gene Silencing and Associated Immunotoxicity of Different Formulations
[0348]In vivo gene silencing studies were performed in the B16F10 lung metastasis model (FIG. 6). Anti-luciferase siRNA formulated in targeted LPH-NPs silenced 80% of the luciferase activity (FIG. 8A). This effect was similar to that of anti-luciferase siRNA formulated in targeted LPD-NPs (FIG. 8A). The other control treatments, including free siRNA, siRNA in non-targeted LPD-NPs or non-targeted LPH-NPs and control siRNA in targeted LPD-NPs or LPH-NPs, showed no RNAi effect. The data shown in FIGS. 5 and 7 suggest that the enhanced gene silencing activity of the targeted LPH-NP is mainly due to the significantly improved tumor uptake. The ED50 of targeted LPH-NP was 0.075 mg / kg (FIG. 8B), the same as the targeted LPD-NP formulation. The optimal dose for maximal gene silencing (80%) was 0.15 mg / kg, and further increases in dosage did not result in greater activity (FIG. 8B). Both the in vitro and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Cytotoxicity | aaaaa | aaaaa |
| Bioactive | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com