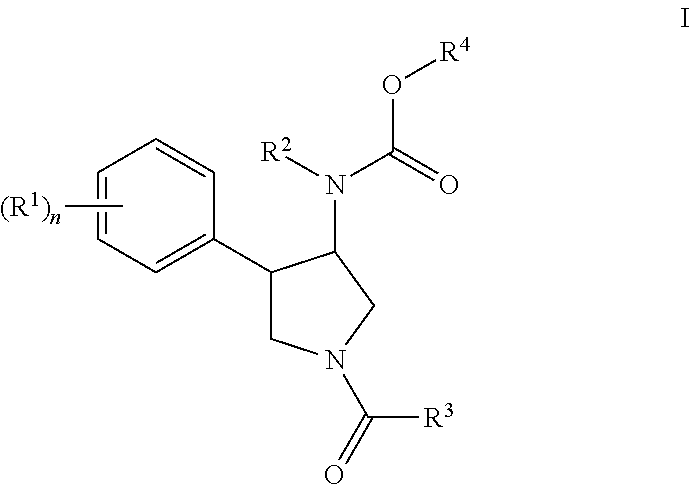
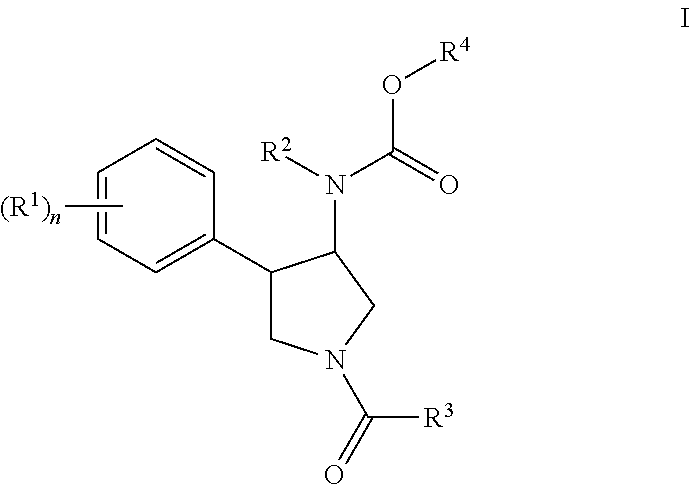
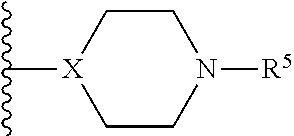
Pyrrolidine compounds
a technology of pyrrolidine and compounds, applied in the field of pyrrolidine compounds, can solve the problems of hampered efforts and lack of knowledge about the cause and nature of this diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
rac-{(3S,4R)-4-(3,4-Dichloro-phenyl)-1-[1-(1-methyl-cyclopropanecarbonyl)-piperidine-4-carbonyl]-pyrrolidin-3-yl}-ethyl-carbamic acid 4-fluoro-phenyl ester
[0254]
a) rac-(3R,4S)-1-Benzyl-3-(3,4-dichloro-phenyl)-4-nitro-pyrrolidine
[0255]A solution of N-(methoxymethyl)-N-(phenylmethyl)-N-(trimethylsilyl)methylamine (32.50 g, 0.135 mol) in CH2Cl2 (70 mL) was added drop wise, over a 30 minutes period, to a stirred solution of 1,2-dichloro-4-((E)-2-nitro-vinyl)-benzene (19.60 g, 0.09 mol) and trifluoroacetic acid (1.54 mL, 0.013 mol) in CH2Cl2 (160 mL) at 0° C. The ice bath was removed, and the solution was stirred at 25° C. for an additional 48 h. It was then concentrated and purification by flash chromatography (SiO2, EtOAc / H 1:6) afforded 25.0 g (79%) of the title compound as a yellow oil. MS m / e: 351.0 (M+H+).
b) rac-(3S,4R)-1-Benzyl-4-(3,4-dichloro-phenyl)-pyrrolidin-3-ylamine
[0256]To a stirred solution of rac-(3R,4S)-1-benzyl-3-(3,4-dichloro-phenyl)-4-nitro-pyrrolidine (11.60 g, 33.0 ...
example 2
rac-{(3S,4R)-4-(3,4-Dichloro-phenyl)-1-[1-(1-methyl-cyclopropanecarbonyl)-piperidine-4-carbonyl]-pyrrolidin-3-yl}-isopropyl-carbamic acid 4-fluoro-phenyl ester
[0263]
a) rac-[(3R,4S)-3-(3,4-Dichloro-phenyl)-4-isopropylamino-pyrrolidin-1-yl]-[1-(1-methyl-cyclopropanecarbonyl)-piperidin-4-yl]-methanone
[0264]To a solution of rac-(3S,4R)-[3-Amino-4-(3,4-dichloro-phenyl)-pyrrolidin-1-yl]-[1-(1-methyl-cyclopropanecarbonyl)-piperidin-4-yl]-methanone (120 mg, 0.28 mmol) in dichloromethane (1 mL) were added acetone (21 uL, 0.28 mmol) and sodiumtriacetoxy-borohydride (72 mg, 0.34 mmol) and acetic acid (16 uL, 0.28 mmol) and the reaction mixture was stirred at ambient temperature for 3 h. It was diluted with dichloromethane and washed with aqueous sodiumhydrogenecarbonate (1M). The organic layer was dried over sodium sulfate and concentrated affording the title compound (95 mg, 72%) as a light yellow foam. MS m / e: 466.3 [M]+.
b) rac-{(3S,4R)-4-(3,4-Dichloro-phenyl)-1-[1-(1-methyl-cyclopropanecarb...
example 3
rac-{(3S,4R)-4-(3,4-Dichloro-phenyl)-1-[1-(1-methyl-cyclopropanecarbonyl)-piperidine-4-carbonyl]-pyrrolidin-3-yl}-isobutyl-carbamic acid 4-fluoro-phenyl ester
[0266]
a) rac-[(3R,4S)-3-(3,4-Dichloro-phenyl)-4-isobutylamino-pyrrolidin-1-yl]-[1-(1-methyl-cyclopropanecarbonyl)-piperidin-4-yl]-methanone
[0267]To a solution of rac-(3S,4R)-[3-Amino-4-(3,4-dichloro-phenyl)-pyrrolidin-1-yl]-[1-(1-methyl-cyclopropanecarbonyl)-piperidin-4-yl]-methanone (150 mg, 0.35 mmol) in dichloromethane (1 mL) were added isobutylaldehyde (39 uL, 0.42 mmol) and sodiumcyanoborohydride (27 mg, 0.42 mmol) and acetic acid (51 uL, 0.88 mmol) and the reaction mixture was stirred at ambient temperature for 3 h. It was diluted with dichloromethane and washed with aqueous sodiumhydrogenecarbonate (1M). The organic layer was dried over sodium sulfate and concentrated affording the title compound (140 mg, 82%) as a light yellow oil. MS m / e: 480.3 [M]+.
b) rac-{(3S,4R)-4-(3,4-Dichloro-phenyl)-1-[1-(1-methyl-cyclopropanecar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com