Liposomal systems comprising sphingomyelin
a technology of sphingomyelin and liposome, applied in the field of liposome technology, can solve the problems of unresolved phenomenon, unfavorable pharmacokinetics, toxic and unwanted side effects, etc., and achieve the effect of reducing bup leakag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
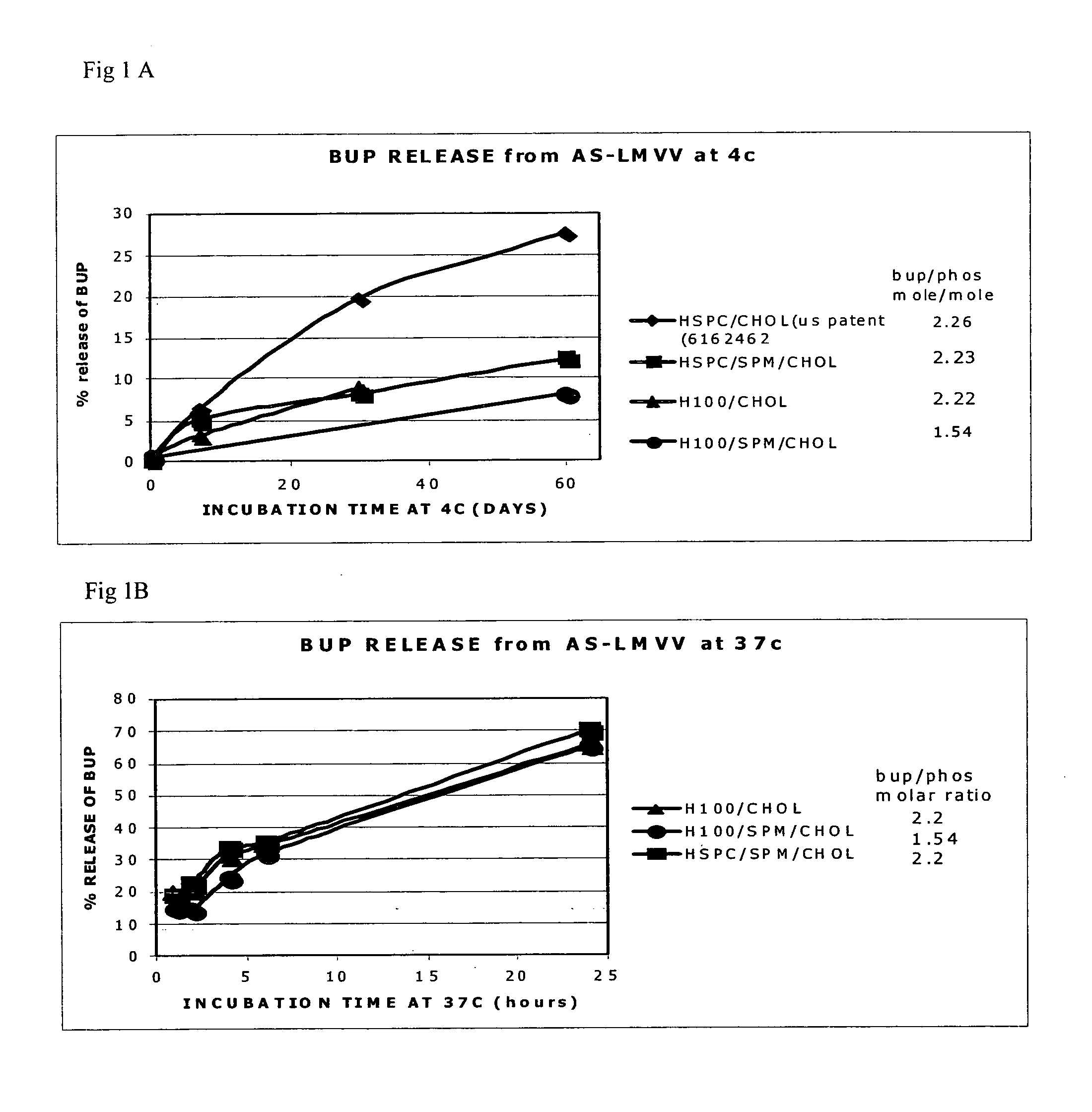
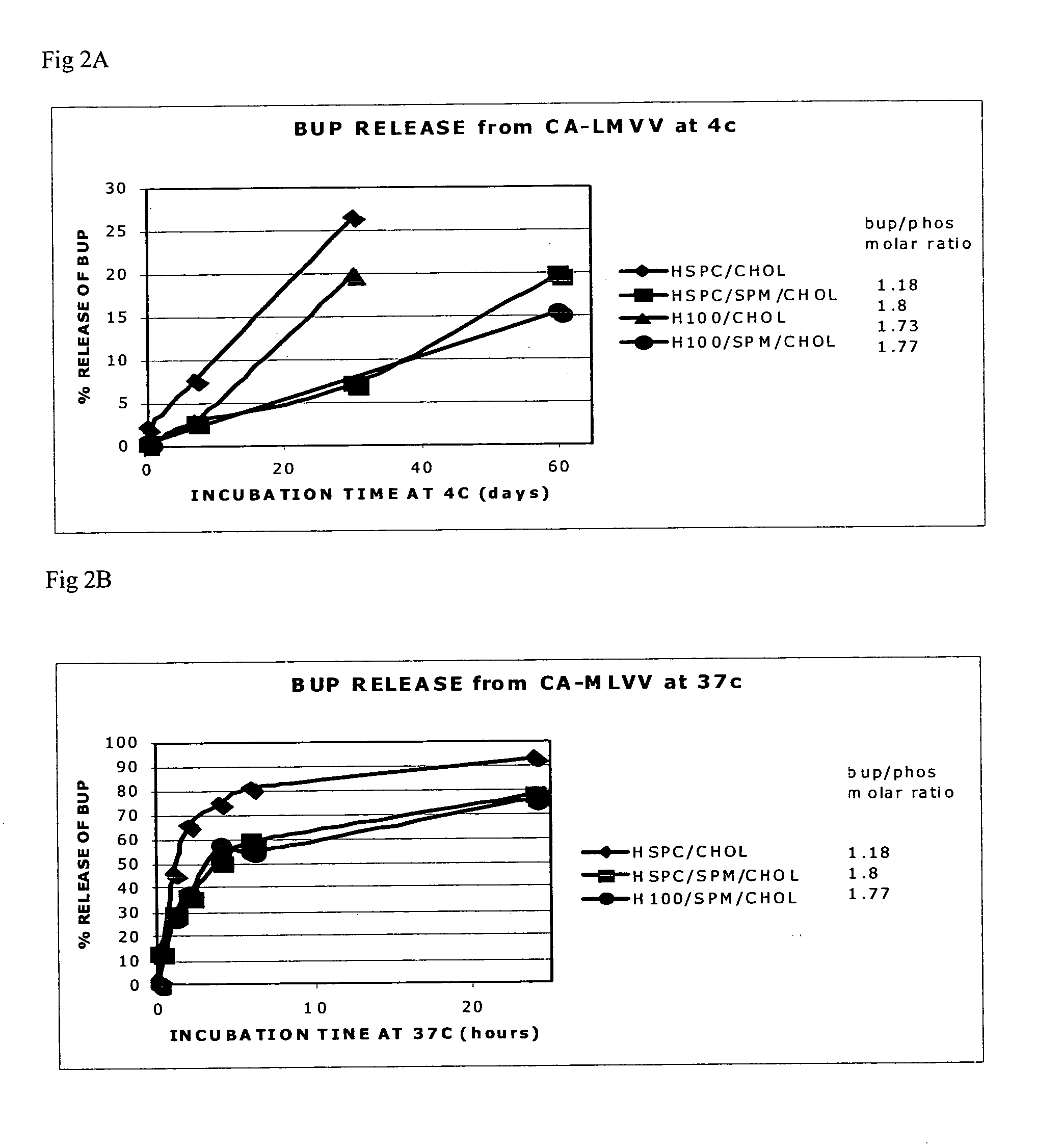
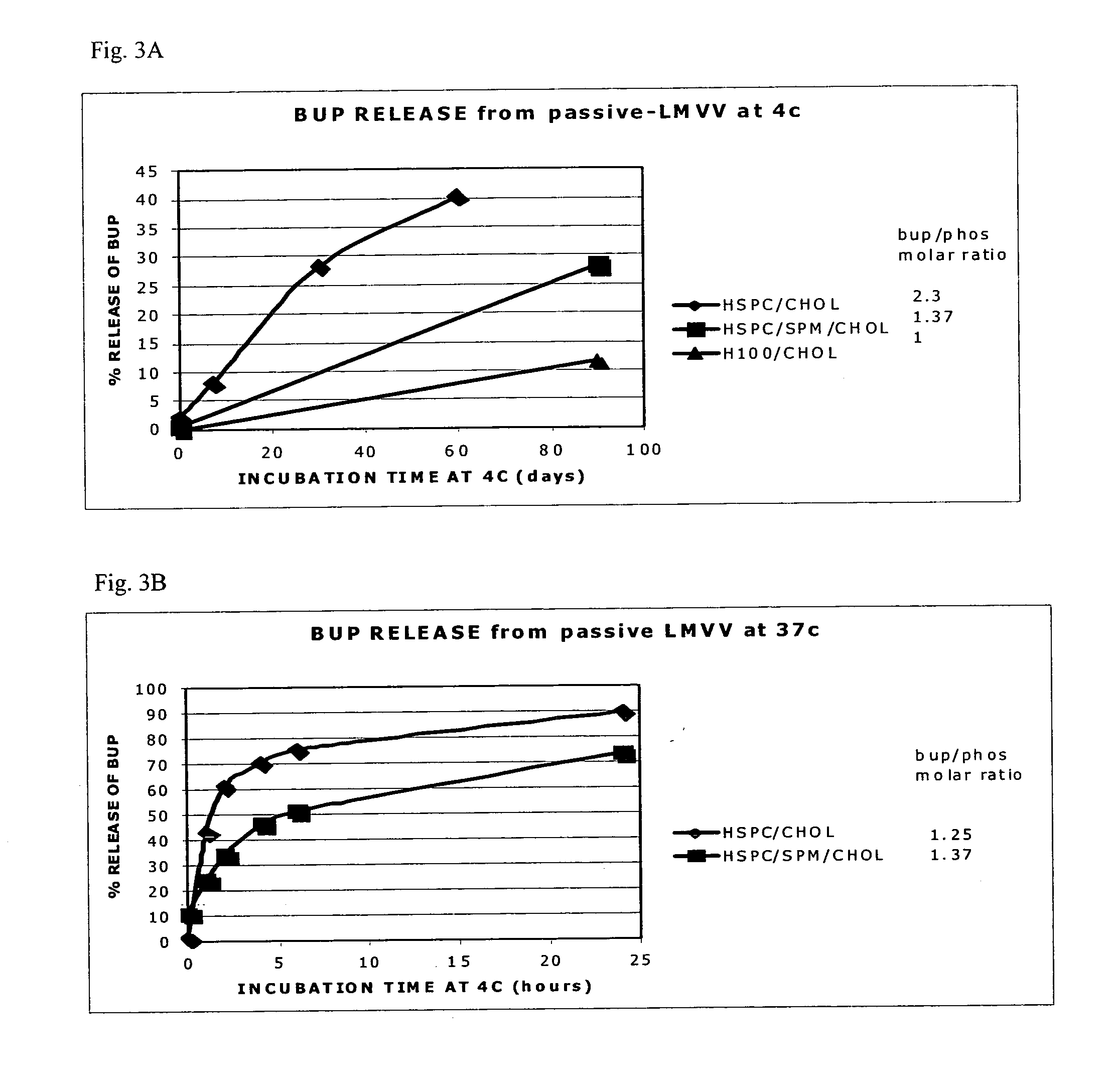
[0021]The present invention is based on the understanding that existing bupivacaine liposomal formulations such as those described in U.S. Pat. No. 6,162,462, and Grant et al. (Grant et al. 2004, ibid.) have a tendency to leak during long term storage at low temperatures which may impose a risk of toxicity when administered to subjects in need of the drug. These bupivacaine liposomal formulations contained high drug to phospholipid ratio (>0.5 mole / mole) in large multivesicular vesicle (LMVV, referred to in U.S. Pat. No. 6,162,462 as giant multivesicular vesicles, GMV), albeit, following storage, a substantial amount of the a priori encapsulated drug was found to be present in the external medium. Thus, a novel liposomal system was designed where the amount of free bupivacaine in the medium external to the liposomes was significantly reduced after long term storage at 4° C., as compared to the hitherto existing bupivacaine liposomal formulations. It was further found that while the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com