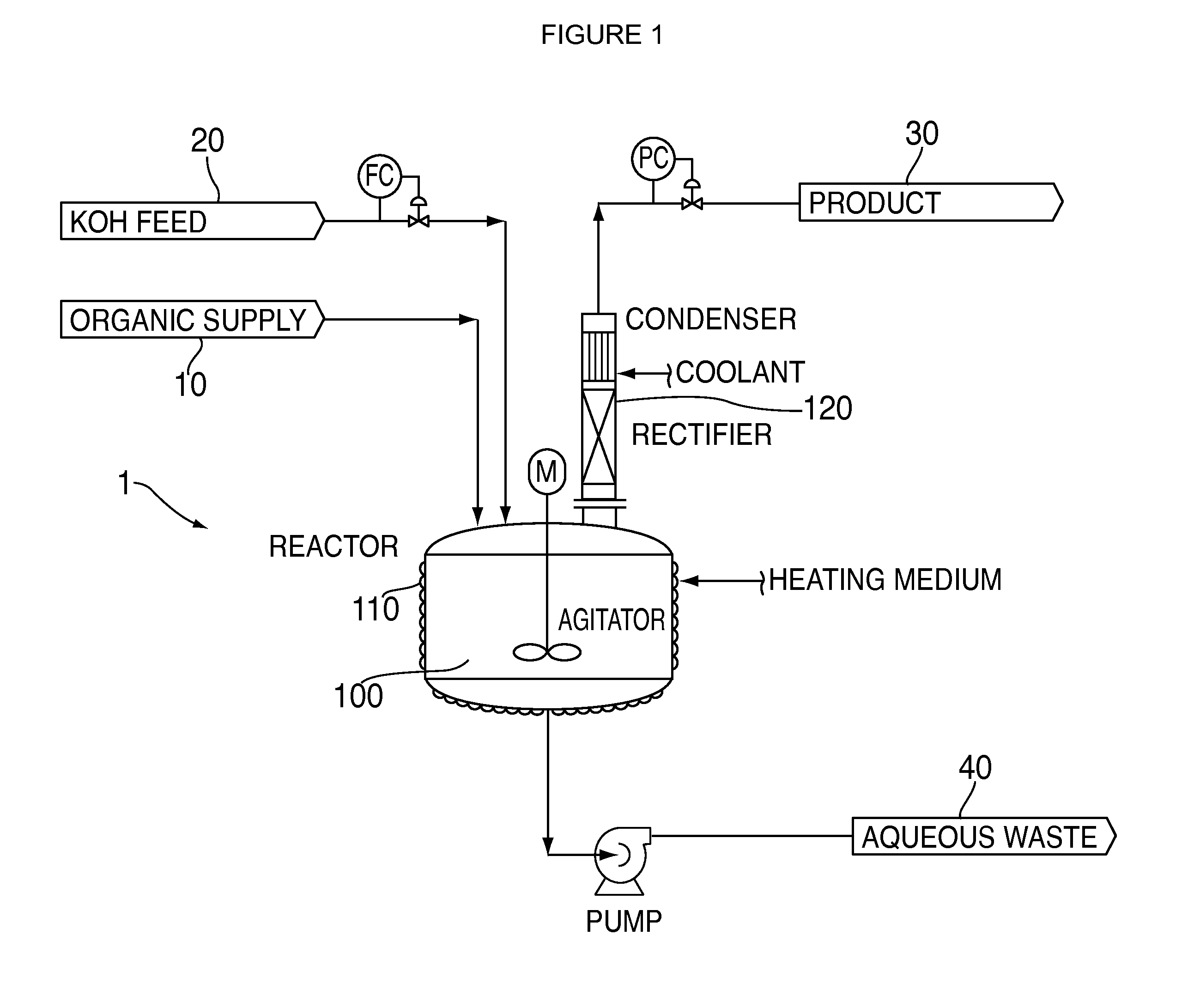
Process for dehydrohalogenation of halogenated alkanes
a technology of halogenated alkanes and dehydrohalogenated alkanes, which is applied in the field of hydrofluoroolefin production processes, can solve the problems and achieve the effect of increasing product yield and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HFO-1225zc from HCFC-235fa
[0021]The same equipment, reactants, and operating conditions were used as described in Comparative Example 1, but for Example 1 the order of addition was changed. The 27.1 lbs of crude organic containing 65% HCFC-235fa organic was put into the reactor first, and heated to the reaction temperature. The rectifying column was then started at total reflux. Next, 38% KOH solution and phase transfer catalyst were fed into the reactor continuously over several hours. The dehydrochlorination reaction proceeded consuming the KOH as it was added leading to a lower pH system, which did not decompose the reactants and product, and allowed recovery of the product overhead.
[0022]The results of this experiment were better than those of comparative example 1. More particularly, the reaction demonstrated a 85% conversion of HCFC-235fa and 77% selectivity to HFO-1225zc.
example 2
HFO-1234ze from HFC-245fa
[0024]The same equipment from Comparative Example 2 was used in Example 2. The reaction was run in a semi-batch mode. About 12500 lbs of 245fa was charged to the reaction vessel. The reactor was then heated to about 60° C. After the desired temperature was achieved, 12000 lbs of 45% KOH was fed to the reactor over a several hour period to produce a desired HFO-1234ze product by dehydrofluorination. As the reaction was initiated the pressure in the reactor was allowed to rise to about 80 psig where it was maintained by use of a pressure control valve. Crude HFO-1234ze exiting the top of the condenser was dried with a desiccant and collected in a chilled tank. The rectifying column and condenser continuously refluxed unreacted 245fa back to the reactor for further processing during the run. After the desired amount of 45% KOH was fed a material balance was performed. The organic yield was 95%, which is quite acceptable for an economical process. Only a slight ...
example 3
HFO-1225ye from HFC-236ea
[0026]The same equipment from Comparative Example 3 is used in Example 3. The reaction is run in a semi-batch mode. About 14400 lbs of 236ea is charged to the reaction vessel. The reactor is then heated to about 60° C. After the desired temperature is achieved, 12000 lbs of 45% KOH is fed to the reactor over a several hour period to produce a desired HFO-1225ye product by dehydrofluorination. As the reaction is initiated the pressure in the reactor is allowed to rise to about 100 psig where it is maintained by use of a pressure control valve. Crude HFO-1225ye exiting the top of the condenser is dried with a desiccant and collected in a chilled tank. The rectifying column and condenser continuously reflux unreacted 236ea back to the reactor for further processing during the run. After the desired amount of 45% KOH is fed a material balance is performed. The organic yield is 95%, which is quite acceptable for an economical process. Only a slight discoloration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com