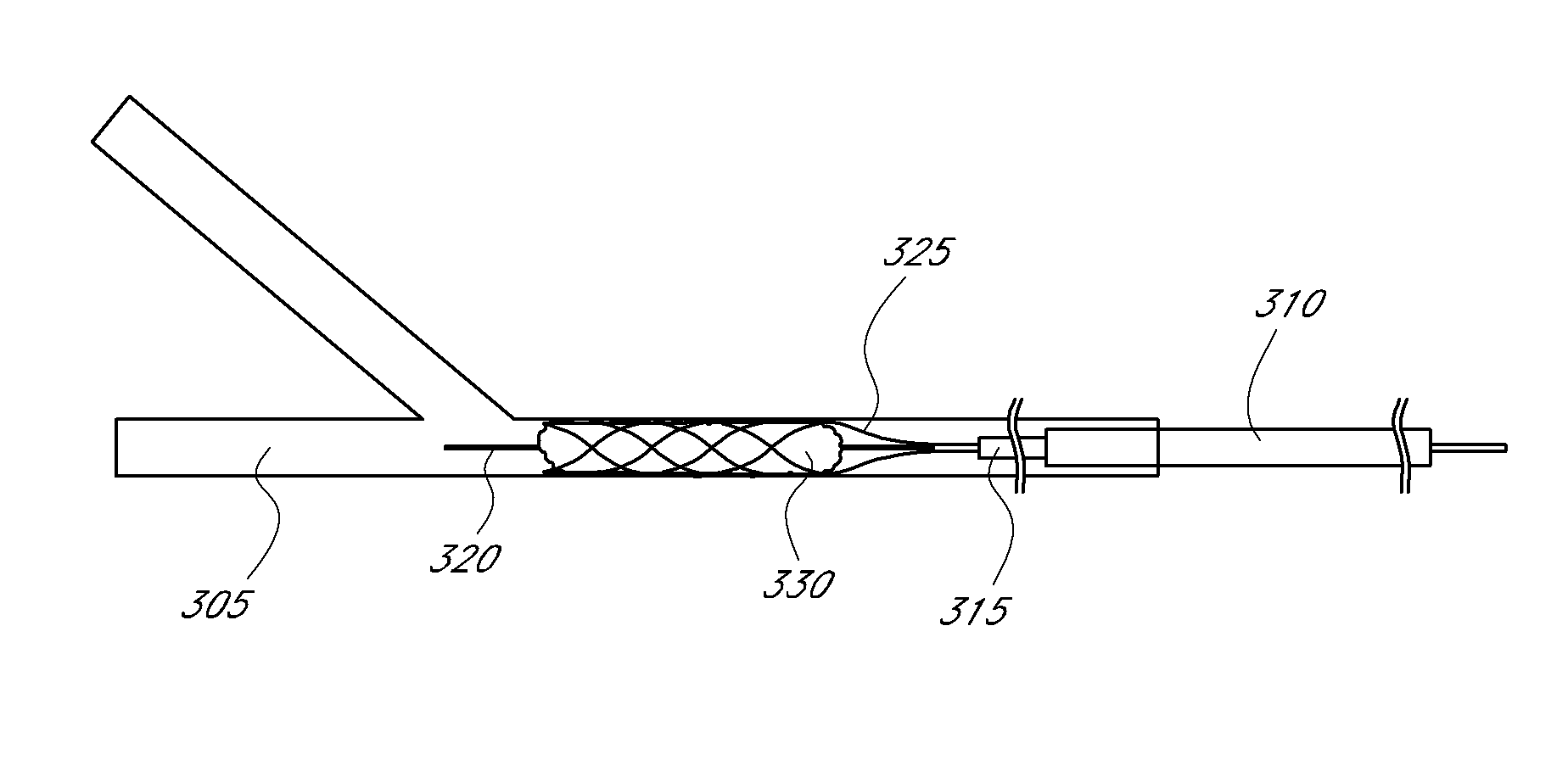
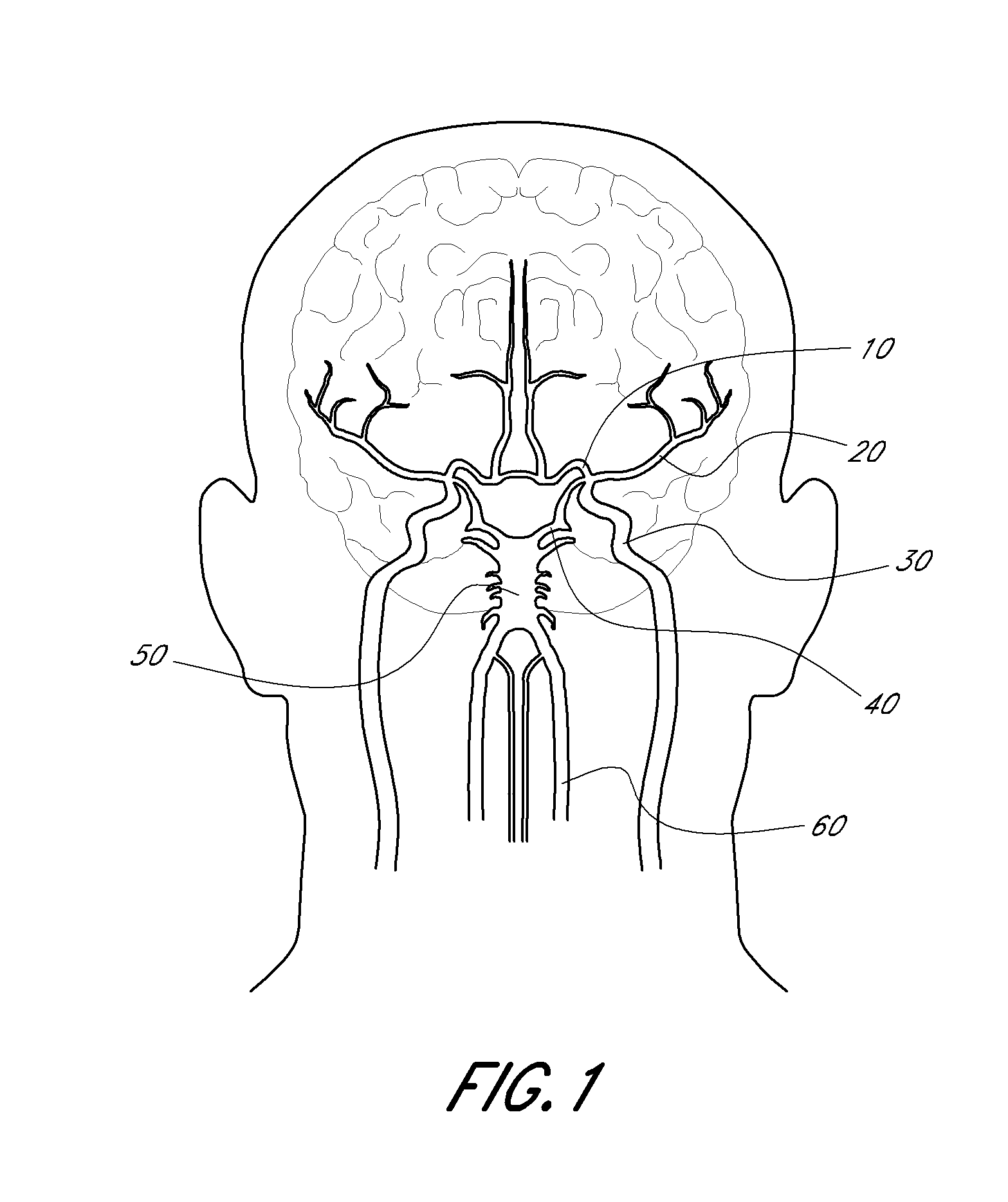
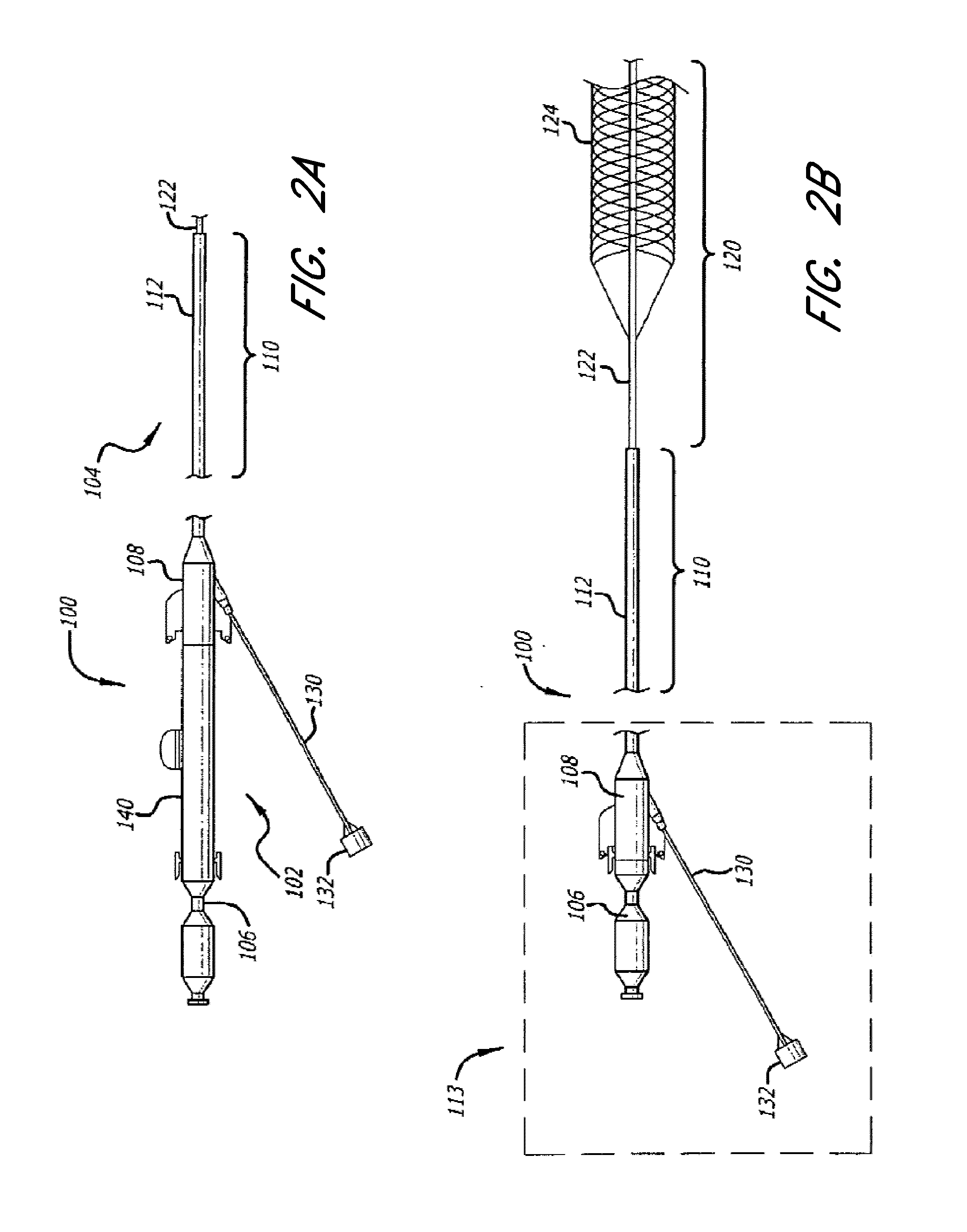
Thrombus management device
a management device and thrombosis technology, applied in the field of thrombosis management devices, can solve the problems of slurred speech, paralysis, loss of memory or brain function, and even death, and achieve the effects of improving the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Stress Test Evaluation for Vessel Tolerance of Revascularization System with Multiple Device Use
[0404]A study was performed to demonstrate that embodiments of multiple revascularization system devices (e.g., expandable tip assemblies) can be challenged and delivered to the target vessel, deployed and then withdrawn from the target vessel in serial fashion without inducing vessel trauma or injury. Angiographic assessment of the target vessels was performed after each device deployment and retrieval to assess performance and outcomes.
[0405]Testing was performed on swine animal models. The swine models were selected because the vascular anatomy and pathological response is comparable to that of the human. Specifically, the internal maxillary and renal arteries are of similar diameter to the human middle cerebral and basilar arteries with diameters of 2.5-3.0 mm respectively. Swine models have been used by neurovascular companies in support of U.S. FDA IDE studies and / or for 510 (k) cle...
example 2
Usability, Safety and Effectiveness of Expandable Tip Assemblies
[0418]A study was performed to determine the usability, safety and effectiveness of embodiments of neurothrombectomy devices comprising expandable tip assemblies designed and configured to facilitate clot removal. Testing was performed on swine animal models. The swine models were selected because the vascular anatomy and pathological response is comparable to that of the human. Swine models have been used by neurovascular companies in support of U.S. FDA IDE studies and / or for 510 (k) clearance. A total of two subject animals and six blood vessels were treated. The blood vessels treated were the left and right ascending pharyngeal, lingual and internal maxillary arteries.
[0419]Embodiments of the expandable tip assemblies or devices were introduced into the target vessels, deployed, pulled through the vessels and retracted into the guide catheter in a manner similar to that described above. This process was repeated up ...
example 3
Radial Force and Cell Characteristics Measurements
[0421]Testing was performed to compare radial force and cell characteristics of various vascular therapy devices, including embodiments of the expandable tip assemblies described herein. The vascular therapy devices tested and / or measured included a NeuroForm3™ device provided by Boston Scientific, an IRIIS™ Plus device provided by MindFrame, an IRIIS™ device provided by MindFrame, a Solitaire™ AB device provided by ev3, and an Enterprise™ device provided by Cordis. The IRIIS™ Plus and the IRIIS™ devices are embodiments of the expandable tip assemblies described herein.
[0422]The following tables illustrate the data collected from the testing. Graphical results of the data from Tables 1 and 2 can be found in FIGS. 6 and 7, respectively, of U.S. Patent Application Publication No. 2010 / 0174309, the entire contents of which has been incorporated by reference herein.
[0423]Table 1 below lists the data obtained from testing performed to det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com