Method for generating replication defective viral vectors that are helper free
a technology of defective viral vectors and gene therapy, applied in the direction of sugar derivatives, biochemistry apparatus and processes, animal husbandry, etc., can solve the problems of development of simple systems, and inability to produce large quantities of pure replication defective viral vectors, so as to prevent the replication and/or packaging of dhlpv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Helper-Free dAAV Vectors
[0125]An hygromycin-sensitive cell line was obtained that expresses the ICP4 gene product. A hygromycin resistant plasmid containing the Epstein-Barr Virus (EBV) origin of replication and the EBNA gene was constructed so as to contain two essential AAV genes, Rep and Cap. This plasmid was then introduced into this cell line. A cell line expressing Rep / Cap and ICP4 was created (i.e., Rep+ / Cap+ / ICP4+ cells) by selecting cells that were hygromycin resistant. A second cell line was prepared in an analogous manner except the cell line did not express ICP4 (i.e., Rep+ / Cap+ / ICP4− cells).
[0126]Rep / Cap are expressed at low levels in the absence of adenovirus sequences, so they are stable within the cell prior to infection. Both the Rep+ / Cap+ / ICP4+ cells and the Rep+ / Cap+ / ICP4− cells were used in the study below.
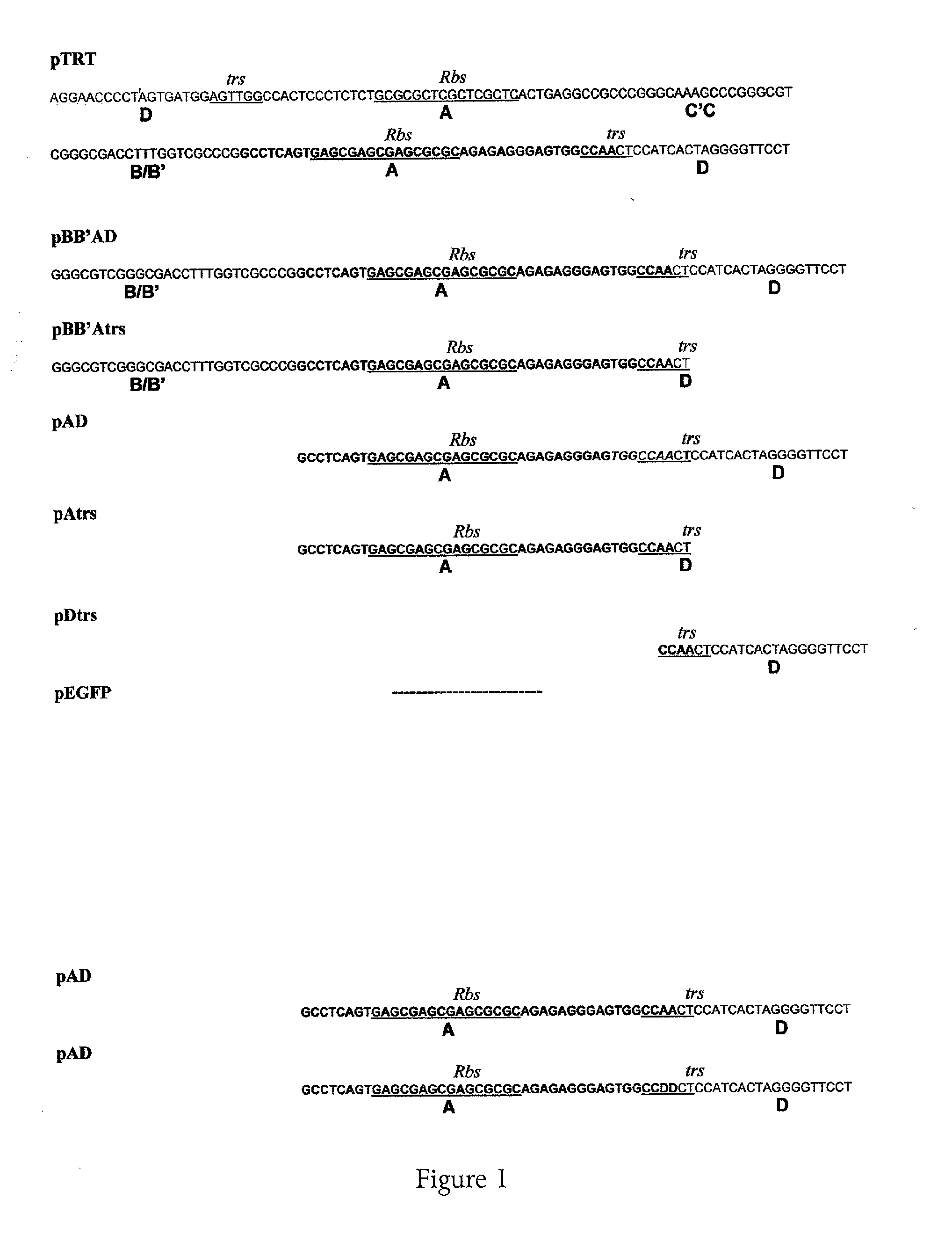
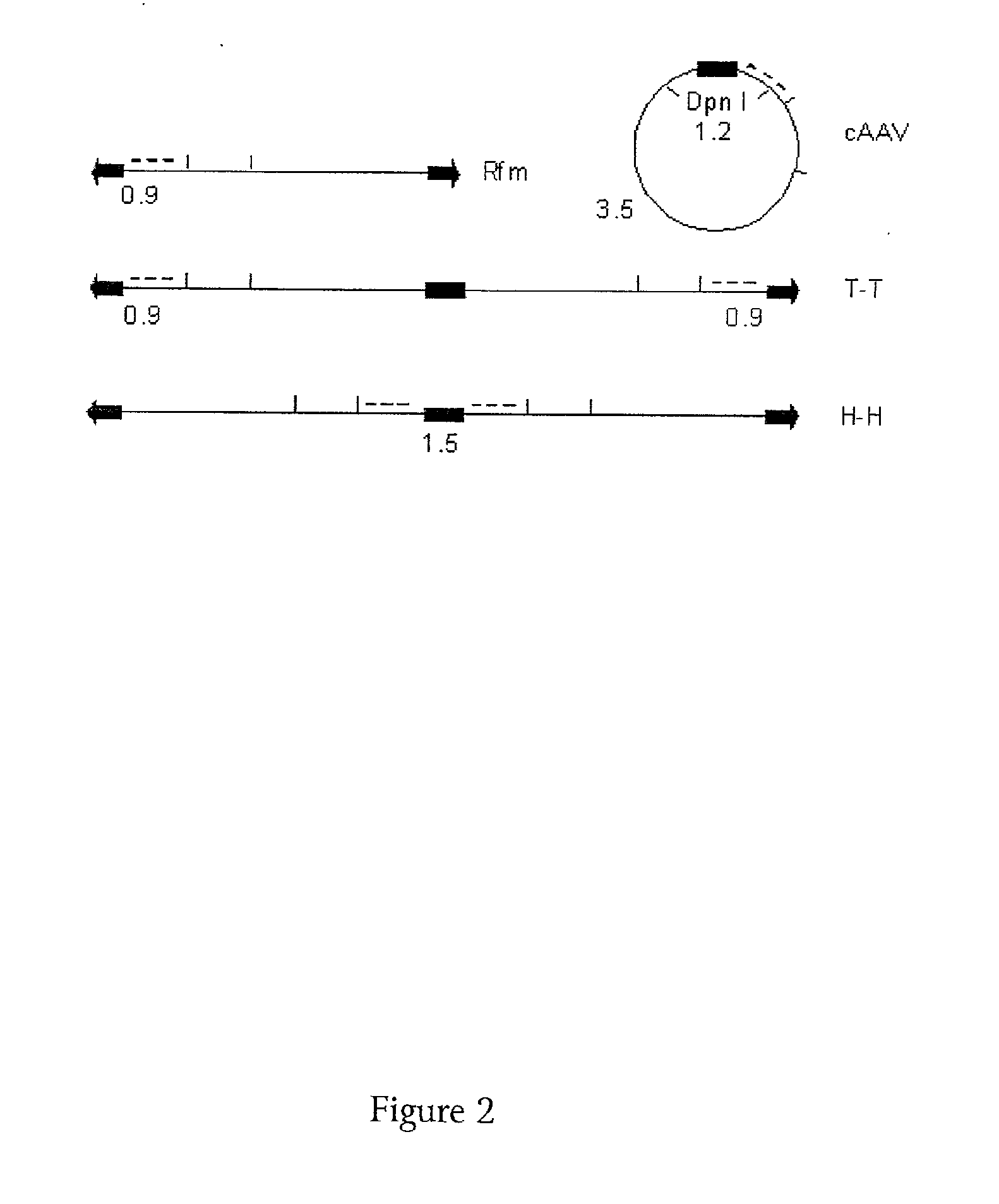
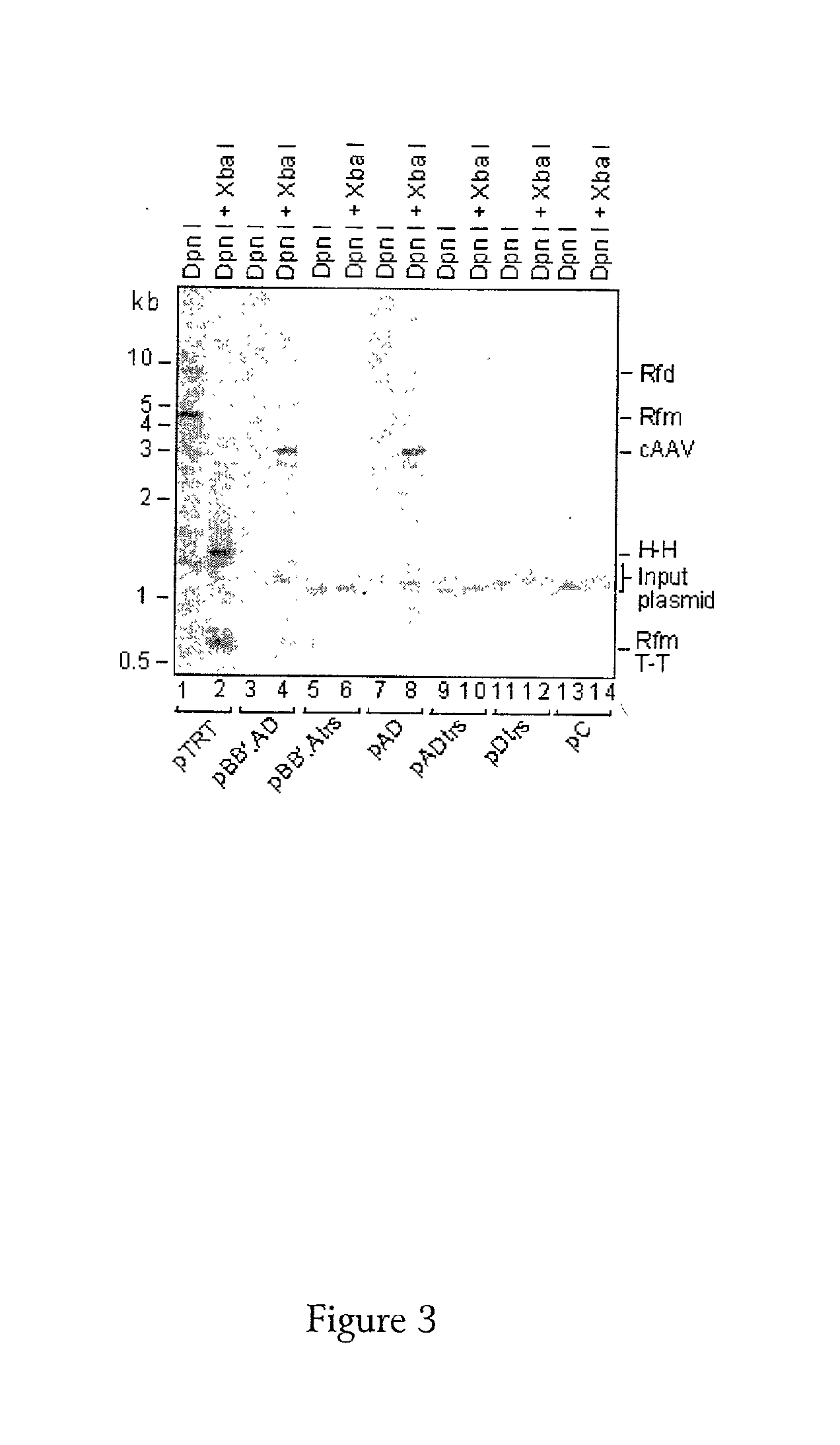
[0127]A defective helper vector was prepared from an HSV virus having a deletion in both copies of the ICP4 gene. Into this viral vector a casset...
example 2
Production of Helper-Free “Gutless” Ad Vectors
[0129]The identical dHSV / Ad helper vector disclosed above, in Example 1 was used with a different cell line for packaging a “gutless” adenovirus (Ad) vector. The gutless Ad vector contains adenovirus termini (harboring origins of DNA replication) and a packaging signal, but no other adenovirus genes.
[0130]A cell line was created which contains a subset of the adenovirus genome inserted into the EBV / EBNA plasmid as described in Example 1 above, to create a stable cell line. These adenovirus sequences contain the adenovirus genome with the E1A, E1B, E2a, E4orf6, and VAI RNA sequences deleted. The deletions were performed in a manner which eliminated any overlap with sequences in the dHSV / Ad helper vector and thereby prevent any possible homologous recombination between the two. In order to retain the essential fiber protein in the cell line, the fiber gene was cloned by PCR, and after deletion of the E4 and part of E3 sequences (which nece...
example 3
Construction of Cell Lines
[0133]One of the most efficient means of producing recombinant AAV, in theory would be to employ a packaging cell line. Unfortunately, heretofore, development of such a cell line has been limited due to the toxicity of the genes required for AAV replication and virion assembly. As disclosed herein, these genes include the AAV rep and cap genes and the adenovirus transcription units: E1A, E2a, E4orf6 and VA RNA.
[0134]The prospects of producing a cell line with a minimal complement of genes appeared to improve with the report that only a subset of these genes (rep, cap, E1 and E4orf6) were sufficient for the generation of high AAV titers (Allen et al. (2000) Mol. Ther. 1(1): 88-95). However, despite extensive efforts, these results could not be confirmed. Indeed, when the rep, cap and E4orf6 coding regions were placed under the control of heterologous promoters, a very poor rAAV titer was obtained (about 0.006 IU / cell).
[0135]Importantly, the addition of a pla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| final concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com