Cation exchange chromatography (methods)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
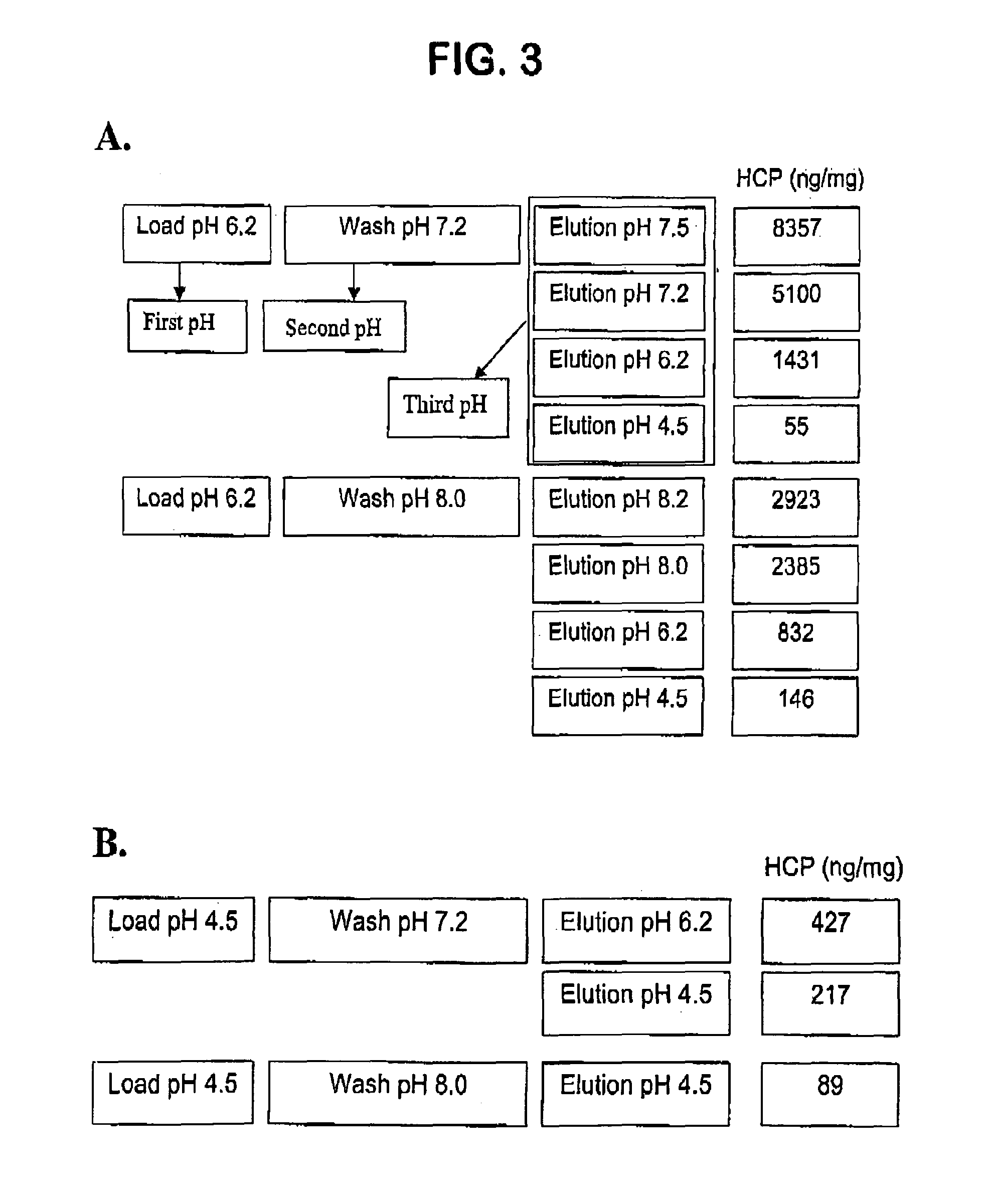
[0042]In this Example a Humab (Humab-1 in table 1) was purified by CEX chromatography using different combinations of load, wash and elution pHs. In all experiments the CEX resin (Poros 50HS (Applied Biosystem)) was packed in a 1 mL column (Φ0.5 cm×H 5 cm).
[0043]In one set of experiments (FIG. 3A), a CEX column was equilibrated with Sodium Phosphate buffer at pH 6.2 and 5.8 mS / cm. A solution of Humab-1 was adjusted to pH 6.2 and 5.8 mS / cm, and loaded onto the column at a concentration of 15 mg / mL. The column was then washed with Sodium Phosphate and Sodium Chloride dual buffer at pH 7.2 or Sodium Phosphate buffer at pH 8.0. After the wash step, the Humab was eluted from the column at pH 8.2, 8.0, 7.5, 7.2, 6.2, or 4.5 with Sodium Phosphate buffer containing sufficient Sodium Chloride to reach the appropriate conductivity for elution.
[0044]In another set of experiments (FIG. 3B), a CEX column was equilibrated with Sodium Citrate and Sodium Phosphate dual buffer at pH 4.5 and 2 mS / cm....
example 2
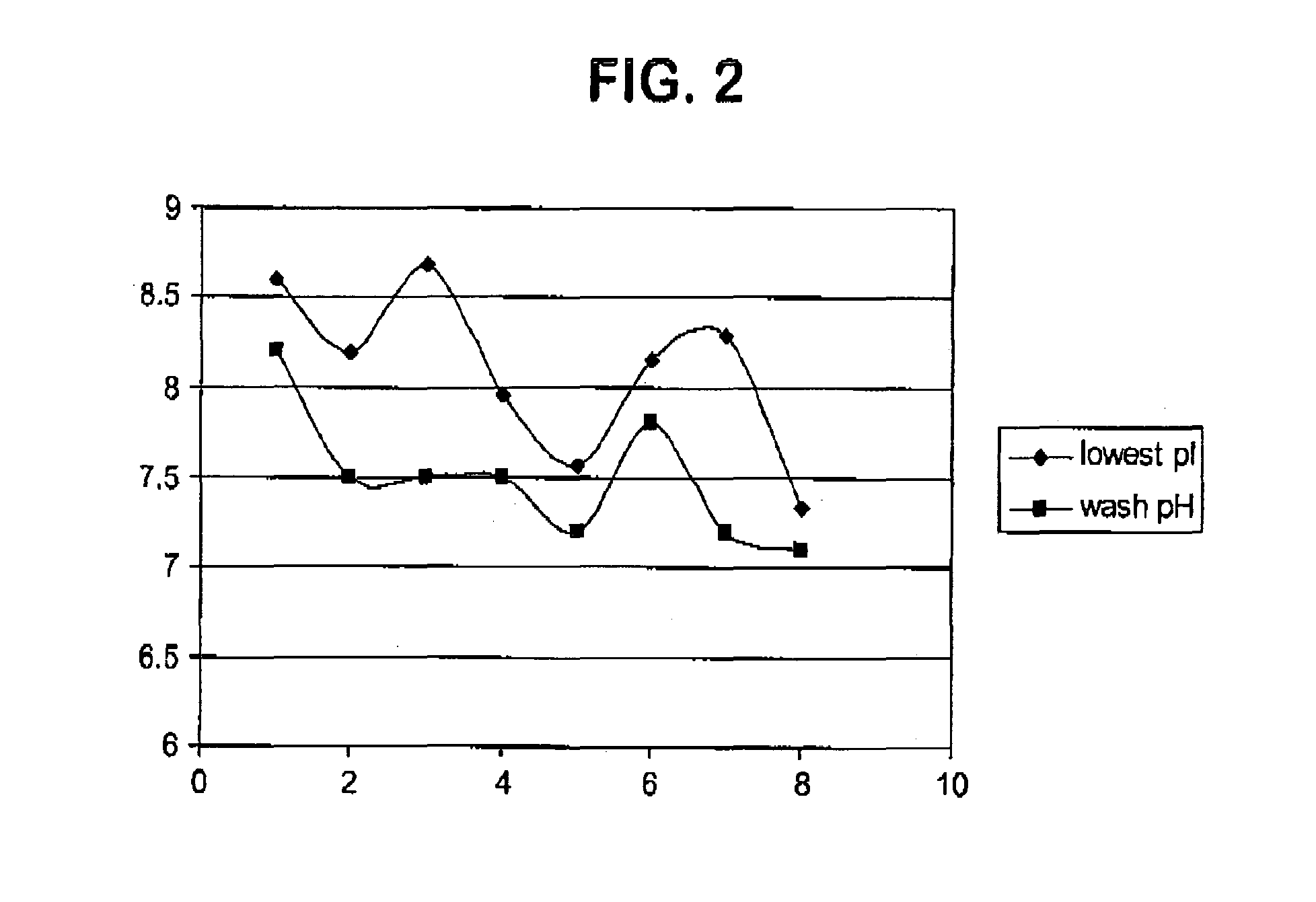
[0046]In this Example IgG1 (Humab-1 in table 1) and IgG4 (Humab-8 in table 1) Humabs were purified using the methods of the invention (see Table 2). A CEX column was equilibrated to pH 6.2. Unpurified bulk containing either Humab-1 or Humab-8 was buffer-exchanged to the same pH and ionic strength as the column equilibration buffer and loaded onto a cation exchange column. The column was then washed with Sodium Phosphate buffer at a pH just below the pI of each Humab. After the wash step, the Humabs were eluted from the column with a buffer comprising either 35 mM Sodium Phosphate, 75 mM NaCl at pH 6.2 (Humab-1) or 75 mM Sodium Phosphate, pH 6.2 (for Humab-8).
[0047]The data in Table 2 show that the CEX chromatography methods of the invention resulted in about a 1000-fold reduction in the HCP contaminant for both Humab-1 and Humab-8.
TABLE 2IgG1 and IgG4 Humab Purification Using the Methods of the InventionHumab-1 (IgG1)Humab-8 (IgG4)(lowest pI 7.96)(lowest pI 7.33)CHO HCPDNACHO HCPDNA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com