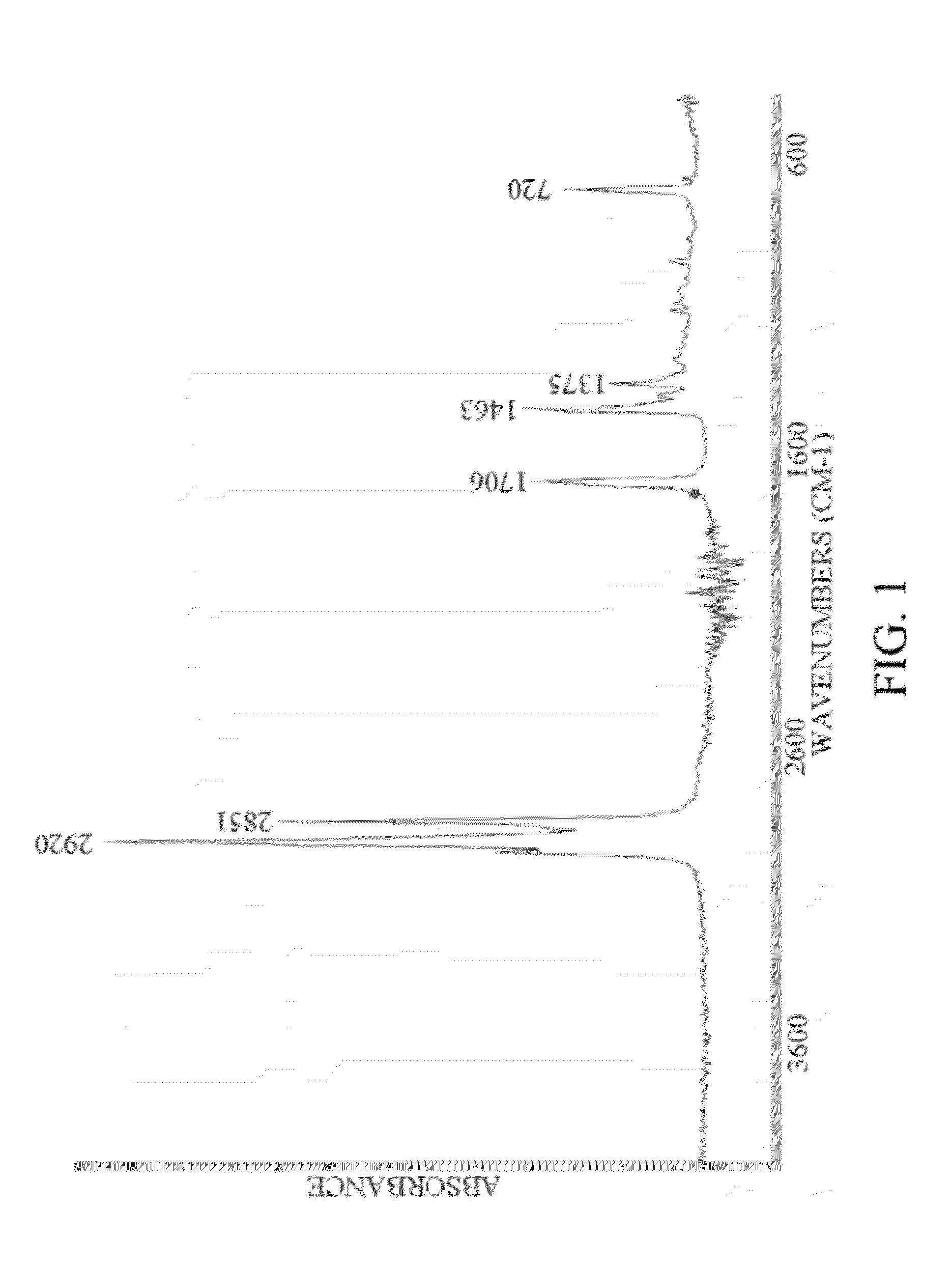
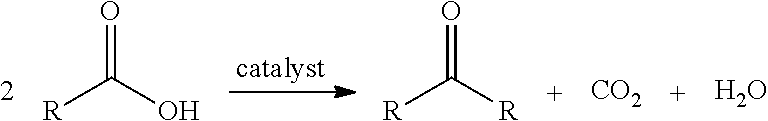
Conversion of fatty acids to base oils and transportation fuels
a technology of transportation fuels and fatty acids, which is applied in the field of conversion of fatty acids to base oils and transportation fuels, can solve the problems of high hydrogen consumption and low molecular weight of some fatty acids by themselves, and achieve the effect of reducing the cost of base oils and reducing the cost of fatty acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Fatty Acid Feeds
[0051]Fatty acid feeds were obtained with properties shown below in Table 1. Diluent was added to the fatty acids to lower the total acid number (TAN) to less than 20 mg-KOH / g feed.
TABLE 1Feed NameCoconut fatty acidStearic acidin n-tridecanein n-octaneAPI Gravity53.2 65.4TAN, mg-KOH / g feed12.7010.2SimDis, wt %0.5 / 5455 / 457250 / 262 10 / 30458 / 461265 / 268 50 / 463 / 269 / 70 / 90464 / 465271 / 272 95 / 99.5466 / 590273 / 689
example 2
Decarboxylation-coupling Dimerization of Coconut Fatty Acid over an Alumina Catalyst
[0052]The decarboxylation-coupling dimerization of coconut fatty acid over an alumina catalyst was conducted at conditions of 680° F. to 730° F., about 0.45 to 1.0 h−1 LHSV and 45 to 60 psig unit pressure. Both whole liquid product (WLP) and Wasson gas samples were taken periodically for inspections. The WLP was also submitted for total acid number test to check the conversion of fatty acid. The results are set forth in Table 2.
TABLE 2Run time, h45141165189957981CAT,° F.680680680680730730LHSV,h−10.980.460.440.480.500.51Pressure, psig455050605050No-lossYield, wt. %C1-400.040.030.020.040.03C5-250°F.0.010.010.0100.080250-550°F.96.7195.5495.3695.639695.97550-700°F.0.450.430.420.370.50.55700°F.+2.122.492.622.522.382.44WLPTAN, mg-KOH / g4.341.011.051.760.240.26SimDis, wt. %0.5 / 5 454 / 458455 / 458455 / 458455 / 459445 / 460449 / 45910 / 30460 / 463460 / 463460 / 464460 / 464462 / 465460 / 46450 / 465 / 465 / 466 / 466 / 467 / 466 / 7...
example 3
Decarboxylation-Coupling Dimerization of Stearic Acid Over an Alumina Catalyst
[0055]The decarboxylation-coupling dimerization of stearic acid over an alumina catalyst was conducted at conditions of 680° F. to 730° F., about 1 to 2 h−1 LHSV and 30 to 50 psig unit pressure. Both whole liquid product (WLP) and Wasson gas samples were taken periodically for inspections. The WLP was also submitted for total acid number test to check the conversion of fatty acid. The results are set forth in Table 4.
TABLE 4Run Time, h11721386110291053CAT, ° F.680680730730730LHSV, h−10.970.971.941.951.95Pressure, psig5045303030No-loss Yield, wt. %C1-40.010.02000 C5-250° F.0.780.730.390.550.57250-550° F.98.0297.6797.1196.6397.43550-700° F.0.930.971.171.171.29700° F.+1.431.832.482.951.95WLPTAN, mg-KOH / g2.972.592.88SimDis, wt. %0.5 / 5246 / 260249 / 262251 / 264250 / 264250 / 264 10 / 30262 / 265264 / 266265 / 268265 / 268265 / 268 50 / 266 / 268 / 270 / 270 / 269 / 70 / 90268 / 269269 / 271271 / 273271 / 272271 / 272 95 / 99.5269 / 910271 / 912273 / 93...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com