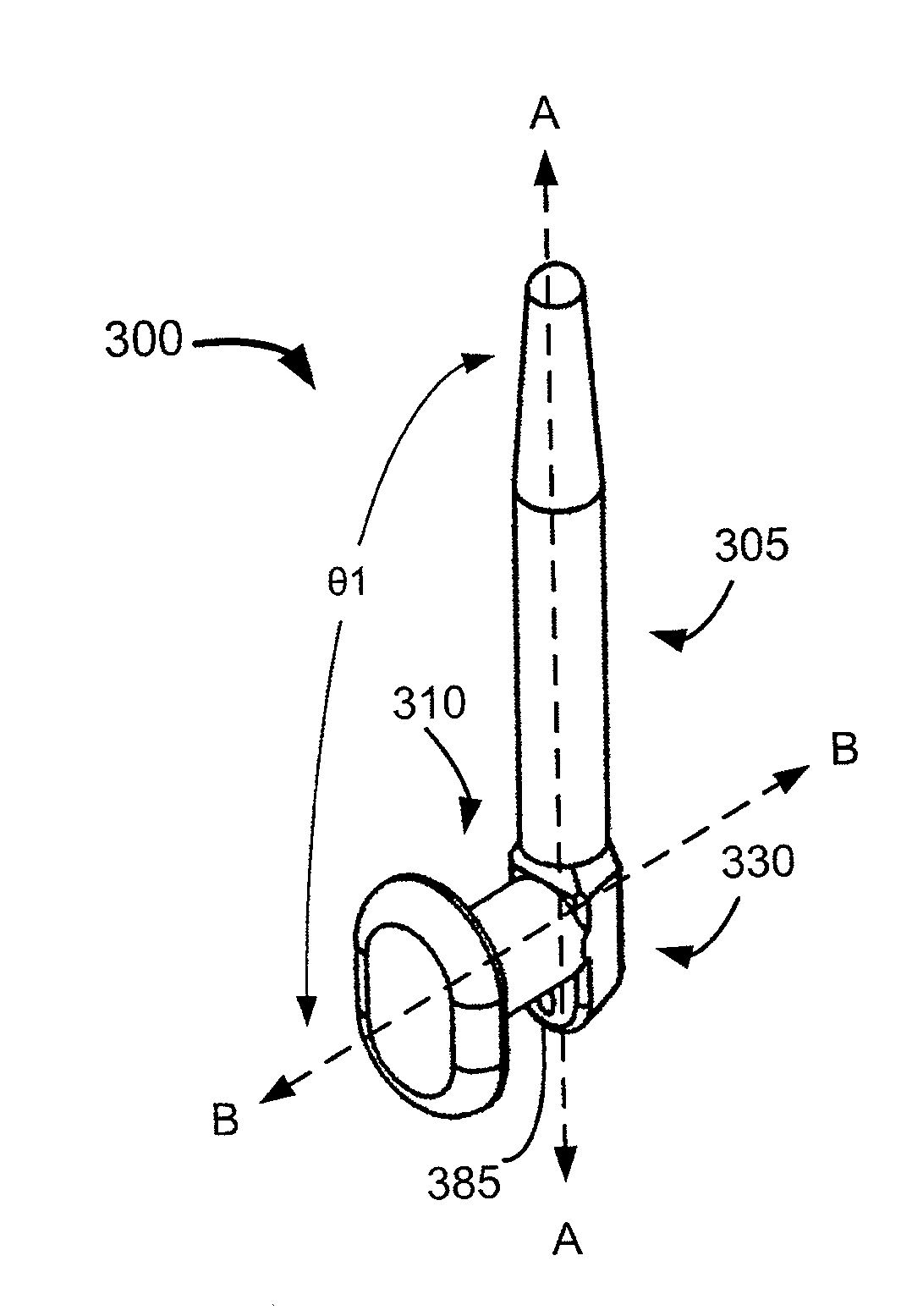
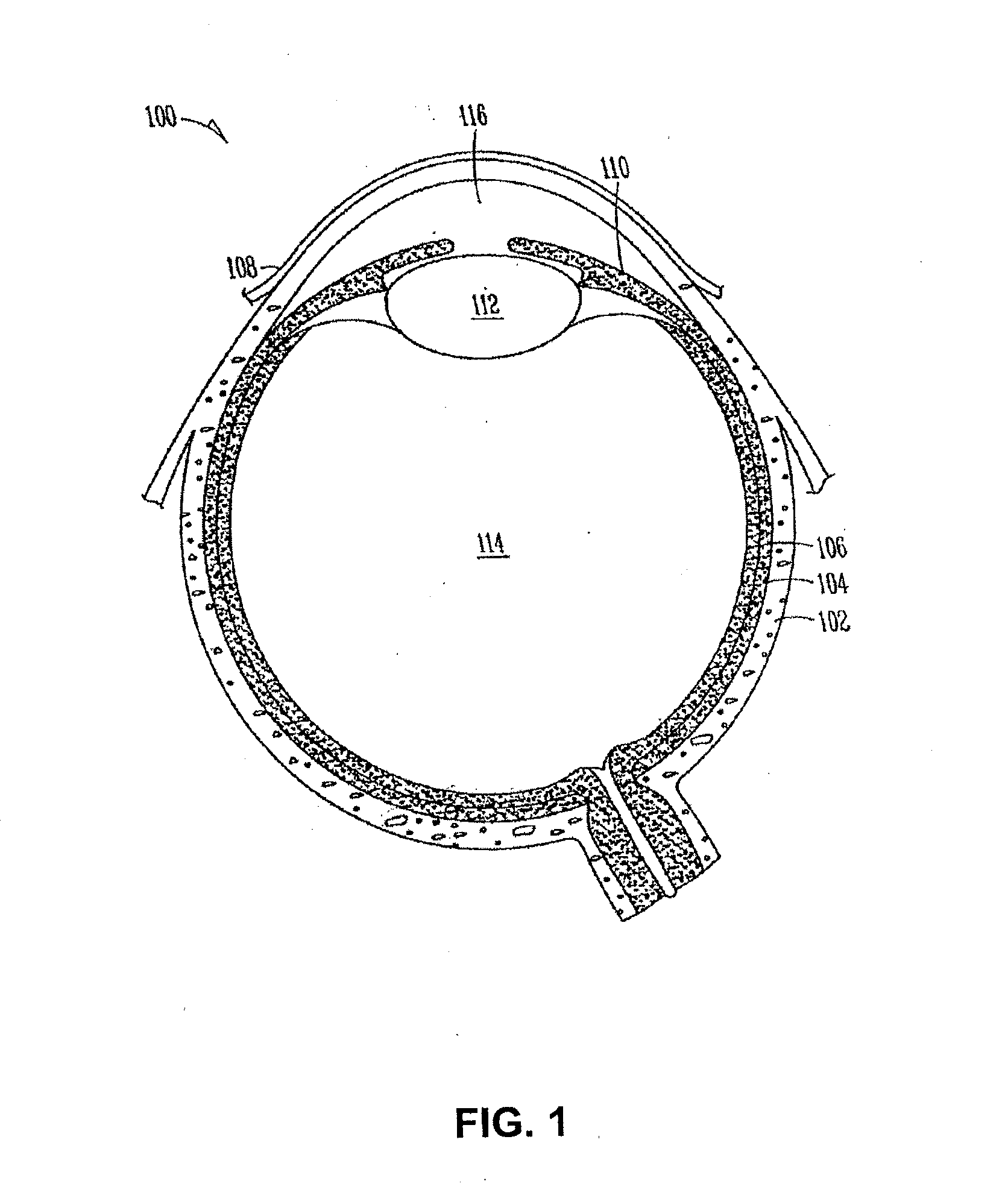
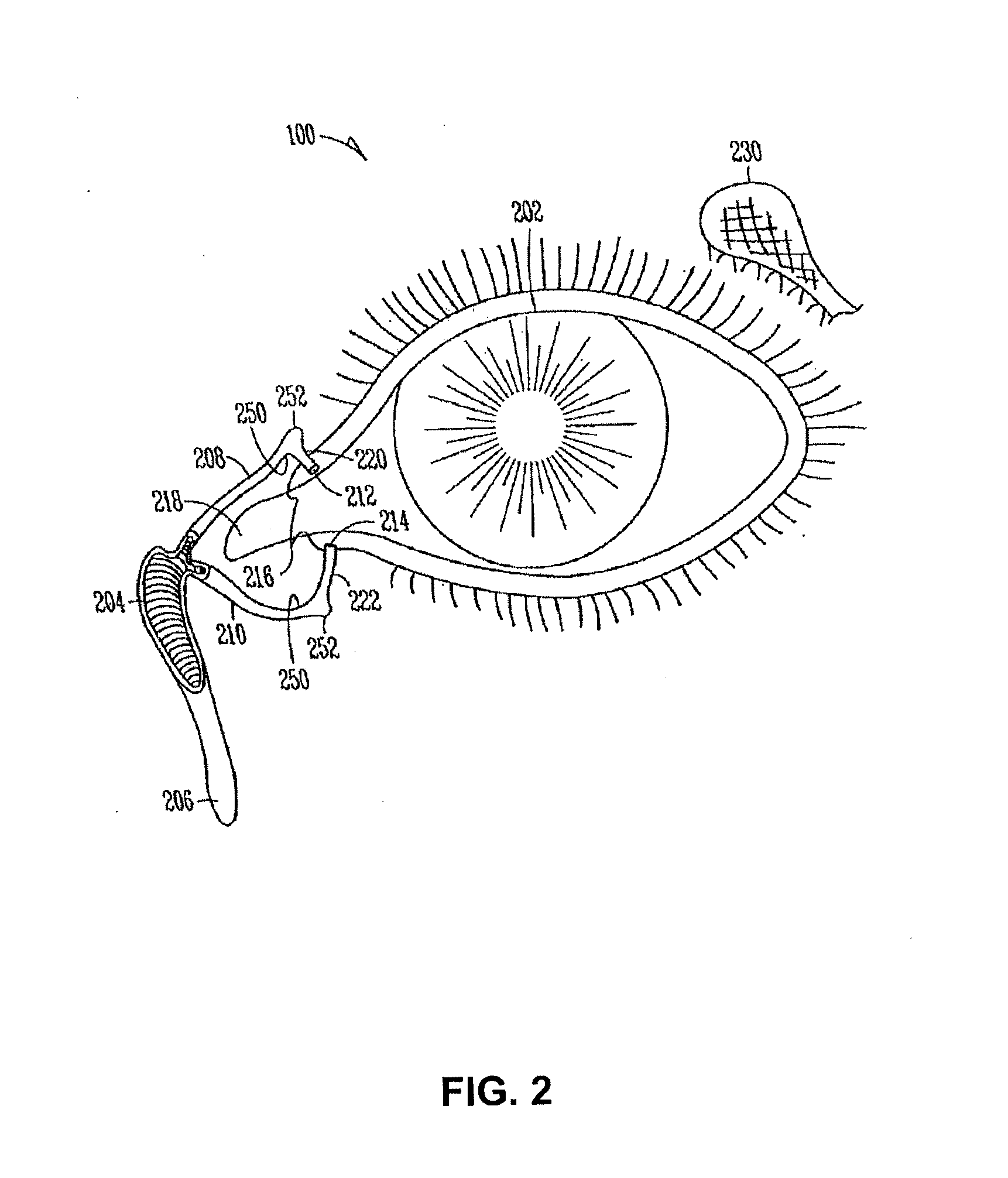
Sustained release delivery of active agents to treat glaucoma and ocular hypertension
a technology of active agents and ocular hypertension, which is applied in the field of ocular diseases, can solve the problems of varied and minimal success in the delivery of latanoprost to the eye using a latanoprost-eluting punctal implant, and achieve the effect of convenient implanting and removal, improving patient comfort and implant retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of Safety and Efficacy of the Latanoprost Punctal Plug Delivery System (L-PPDS) Containing Latanoprost
[0228]A Phase II, open-label, clinical study was conducted in human subjects with ocular hypertension (OH) or open-angle glaucoma (OAG) to evaluate safety and efficacy of the latanoprost punctal plug delivery system (L-PPDS).
[0229]The Phase II trial featured simultaneous placement of punctal plugs in both the upper and lower puncta for delivery of a daily drug load with a goal of enabling comparable clinical outcomes to that of daily administered Xalatan® eyedrops. The overall objective was a mean reduction in IOP of 5 mm Hg or greater. The primary endpoint in this Phase II study was a mean change in IOP from baseline (measured as mm Hg) at 2 weeks. Secondary endpoints were the mean change in IOP from baseline at 4 weeks and mean percentage change in IOP at 2 weeks and 4 weeks. A total of 95 ITT (Intent to Treat) subjects were included in the L-PPDS treatments in this stu...
example 2
Comparison of Clinical Studies of the L-PPDS at Different Dosages
[0245]A number of clinical studies have been conducted to assess the safety, efficacy, and dosing for L-PPDS treatment in more than 300 human subjects with OH or OAG. These studies have investigated the preliminary safety and efficacy of L-PPDS over the dose range of 3.5 to 95 μg per eye, primarily delivered via an L-PPDS positioned in the lower puncta. See, for example, ClinicalTrials.gov Identifier: NCT00967811, “An Open-Label, Phase 2 Study of Different Formulations (E1 and E2) of the Latanoprost Punctal Plug Delivery System (L-PPDS) in Subjects With Ocular Hypertension (OH) or Open-Angle Glaucoma (OAG),” Study Start Date: August 2009, (other study ID number: PPL GLAU 07), incorporated herein by reference; and ClinicalTrials.gov Identifier: NCT01037036, “An Open-Label, Phase 2 Study of the Latanoprost Punctal Plug Delivery System (L-PPDS) With Adjunctive Xalatan® Eye Drops in Subjects With Ocular Hypertension (OH) o...
example 3
Method of Preparation L-PPDS (95 μg) Cores
[0247]NuSil Silicone MED6385 part A was stirred for a minimum of 5 minutes, and 63 mg of which was weighed and transferred onto a glass slide. To the same glass slide was added Latanoprost (obtained from Everlight Chemical, Taipei, Taiwan) (48 mg), dimyristoylphosphatidylcholine (DMPC) (9 mg) and NuSil Med6382 crosslinker (2.4 mL). Using a 0.5 μL Hamilton Syringe, Nusil MED6385 part B (0.348 μL) was transferred directly onto a mini spatula. The latanoprost, NuSil MED6382 crosslinker, NuSil Silicone MED-6385 part B and DMPC were mixed together for 2-5 minutes to form a homogenous mixture. The resulting mixture was combined with NuSil Silicone MED6385 part A and was mixed for another 2 minutes to form a homogenous mixture, which was immediately transferred into a previously prepared syringe assembly. The syringe was then attached to polyimide tubing (0.024″ OD) by way of an adapter. The polyimide tubing was kept at a temperature of 4° C. by wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| intraocular pressure | aaaaa | aaaaa |
| intraocular pressure | aaaaa | aaaaa |
| intraocular pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com