Hexon isolated from simian adenovirus serotype 19, hypervariable region thereof and chimeric adenovirus using the same
a technology of chimeric adenovirus and simian adenovirus, which is applied in the field of hexon, can solve the problems of adenoviruses infecting the liver, antibodies reducing the efficiency of viral replication, and the generation of antigenic epitopes of adenoviral capsids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
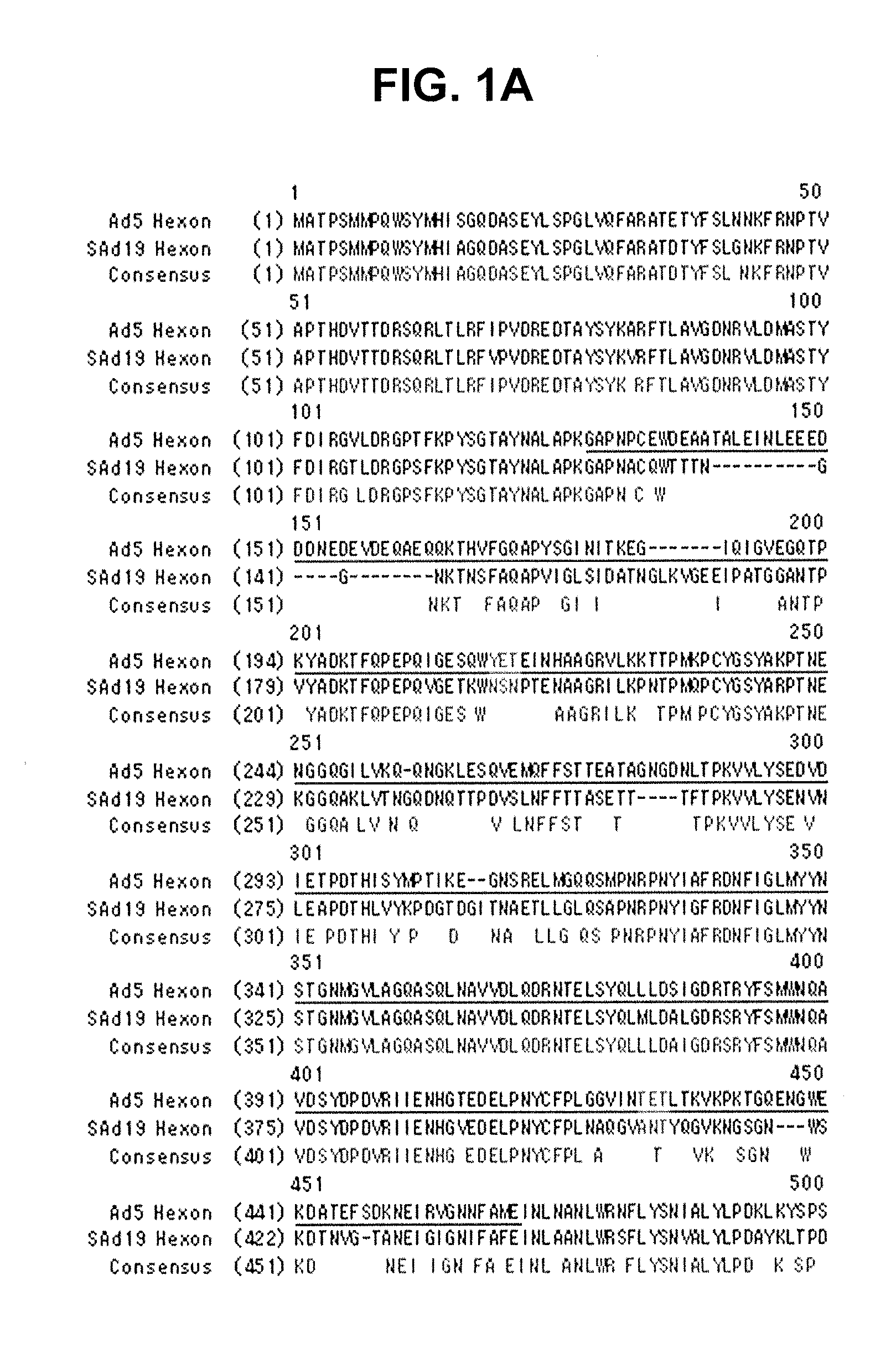
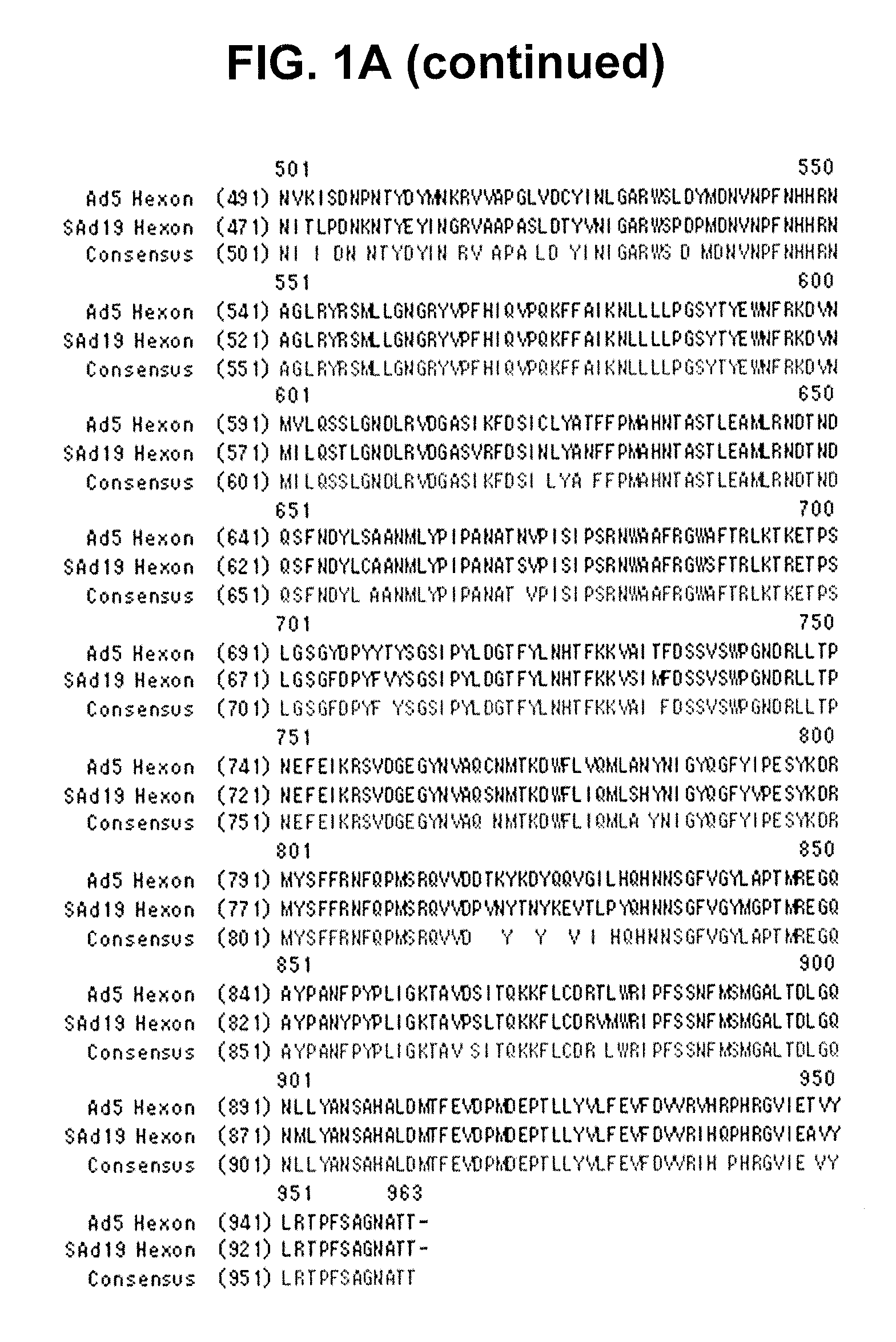
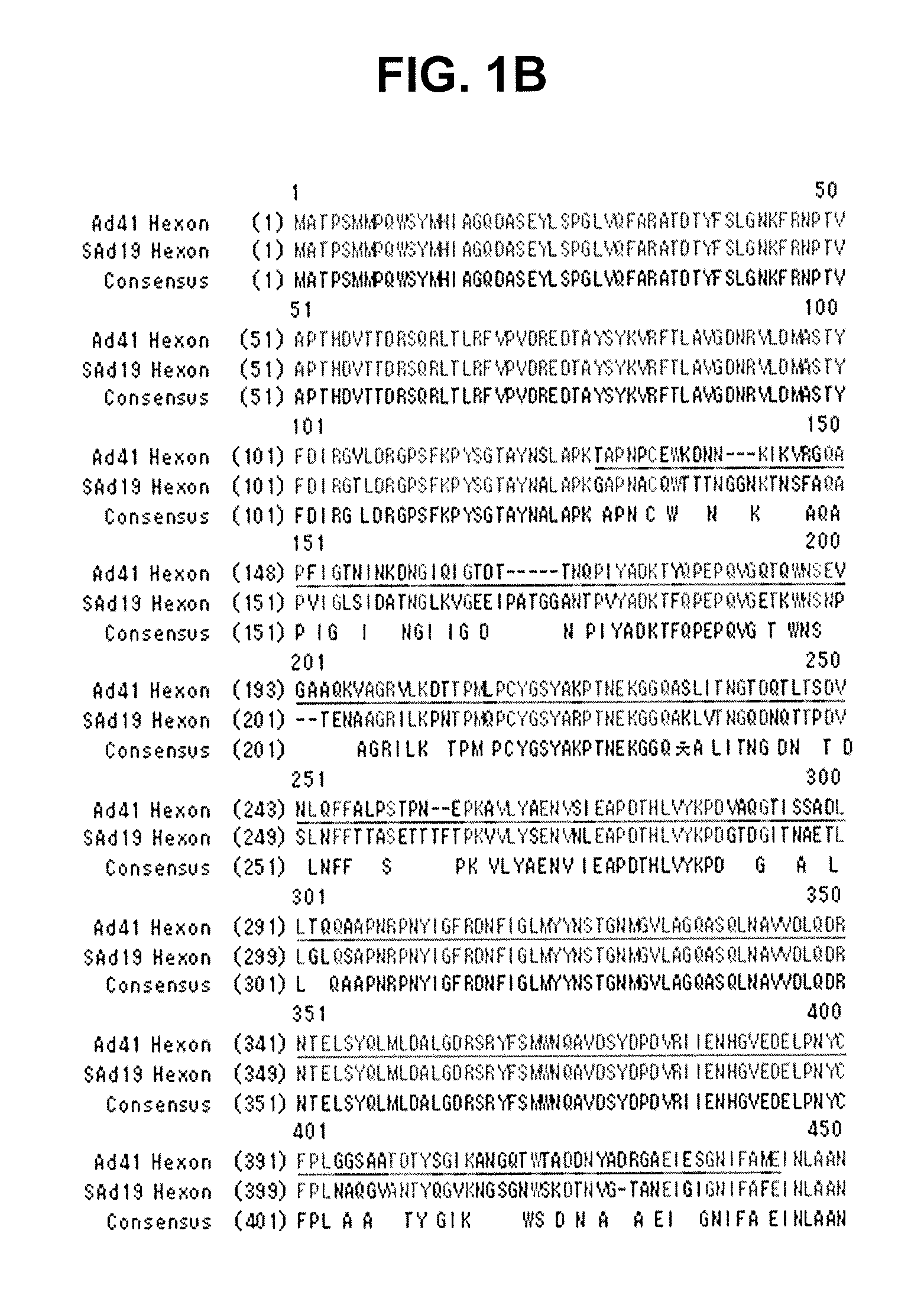
Identification of Hexon Gene of SAd19
[0081]A hexon gene was amplified from SAd19 using PCR, cloned into a pGEM-T easy vector, and sequenced with an ABI automatic DNA sequencer.
Identification of Hexon Gene
[0082]The genome of SAd19 was isolated using a DNeasy Tissue Kit (QIAGEN, Germany) and used as a template for the amplification of a hexon gene via PCR with a primer set of SEQ ID NOs: 1 and 2. The amplified hexon gene was purified by Wizard® SV Gel and PCR Clean-Up system (Promega, WI, USA) and inserted into a pGEM-T easy vector (Promega, WI, USA) with the aid of T4 DNA ligase (Roche, Switzerland). The resulting recombinant vector was named pGEM-SAd19 Hexon vector. After transformation of E. coli cells with the vector, the vector DNAs were extracted from 10 clones of the transformants and sequenced by employing an ABI automatic DNA sequencer. Among the 10 base sequences was selected the one which had the highest sequence homology with others, thereby determining the nucleotide seq...
example 2
Preparation of Chimeric Adenovirus with Hexon of SAd19 Substituted Therein
[0084]A shuttle vector for exchanging a hexon gene was constructed and named pHex vector, which is carrying left- and right-extended regions of hexon gene for homologous recombination. The hexon gene of SAd19 was cloned into unique restriction site of pHex vector, locating between left- and right-extended arms of hexon to afford a recombinant vector, named pHex-SAd19 Hexon. SphI-linearized pHex-SAd19 Hexon was subject to homologous recombination with AsiSI-linearized pAd H5—8DS in BJ5183 (Stratagene, CA, USA) to give a SAd19 Hexon-carrying recombinant vector, named pAd H5 / S19—8DS. After being linearized with Pad, the pAd H5 / S19—8DS was transfected into A549 cells to generate novel chimeric adenovirus with the hexon of SAd19 anchored therein (Ad H5 / S19—8DS).
Construction of Shuttle Vector
[0085]A shuttle vector suitable for the substitution of a hexon gene through homologous recombination was constructed. In thi...
example 3
In Vitro Study of Chimeric Adenovirus Having the Hexon of SAd19
[0096]Ad H5 / S19—8DS virus was in vitro assayed for selectivity for lung cancer by comparing expression levels of E1A and LK8 in the lung cancer cell line A549 and the normal cell line MRCS both of which were infected at various MOIs with the virus. In addition, Ad H5 / S19—8DS virus was measured for affinity for the coagulation factors which mediate blood-circulating virus to liver.
MOI-dependent E1A Expression
[0097]A549 lung cancer cells and MRCS normal cells, which were both grown at about 80% confluency in 6-well plates, were infected with Ad H5 / S19—8DS virus at an MOI of 100, 25, 10 and 1. After incubation at 37° C. for 24 hrs, the cells were harvested by centrifugation at 3,000 rpm for 5 min, suspended in 1×SDS-PAGE buffer (50 mM Tris(pH6.8), 2% SDS, 100 mM dithiothreitol, 0.1% bromophenol blue, 10% Glycerol), heated at 100° C. for 5 min in a water bath, and centrifuged at 10,000 rpm for 2 min. The resulting supernata...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com