Release reagent for vitamin d compounds
a technology of vitamin d and reagents, which is applied in the field of vitamin d release reagents, can solve the problems of vitamin d having a relatively short biological half-life in the circulation, affecting the d status of patients, and the measurement of the vitamin d level itself is of little benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assays for the Detection of 25-hydroxyvitamin D
[0158]Commercial assays are used according to the manufacturer's instructions. The 25-hydroxyvitamin D determinations are carried out by means of HPLC (test for 25(OH)vitamin D3, from the “Immundiagnostik” Company, Bensheim, order No. KC 3400) or by means of LC-MS / MS (Vogeser, M. et al., Clin. Chem. 50 (2004) 1415-1417) as described in the literature.
[0159]The preparation of the ingredients and the general test procedure for a new test is described in the following:
[0160]1.1 Synthesis of hydroxyvitamin D2-3-2′-cyanoethyl ether
[0161]20.6 mg (50 limol) 25-hydroxyvitamin D2 (Fluka No. 17937) is dissolved in a 25 ml three necked round bottom flask with an internal thermometer in 10 ml dry acetonitrile under an argon atmosphere. 1.5 ml tert.-butanol / acetonitrile (9:1) is added to the solution and cooled to 6° C. in an ice bath. Subsequently 820 μl of an acrylonitrile solution (86 μl acrylonitrile in 1.0 ml acetonitrile) is added and stirred ...
example 2
Comparison of Carbonate Ester to a Metal Salt, a Phosphate Buffer and a Carbonate
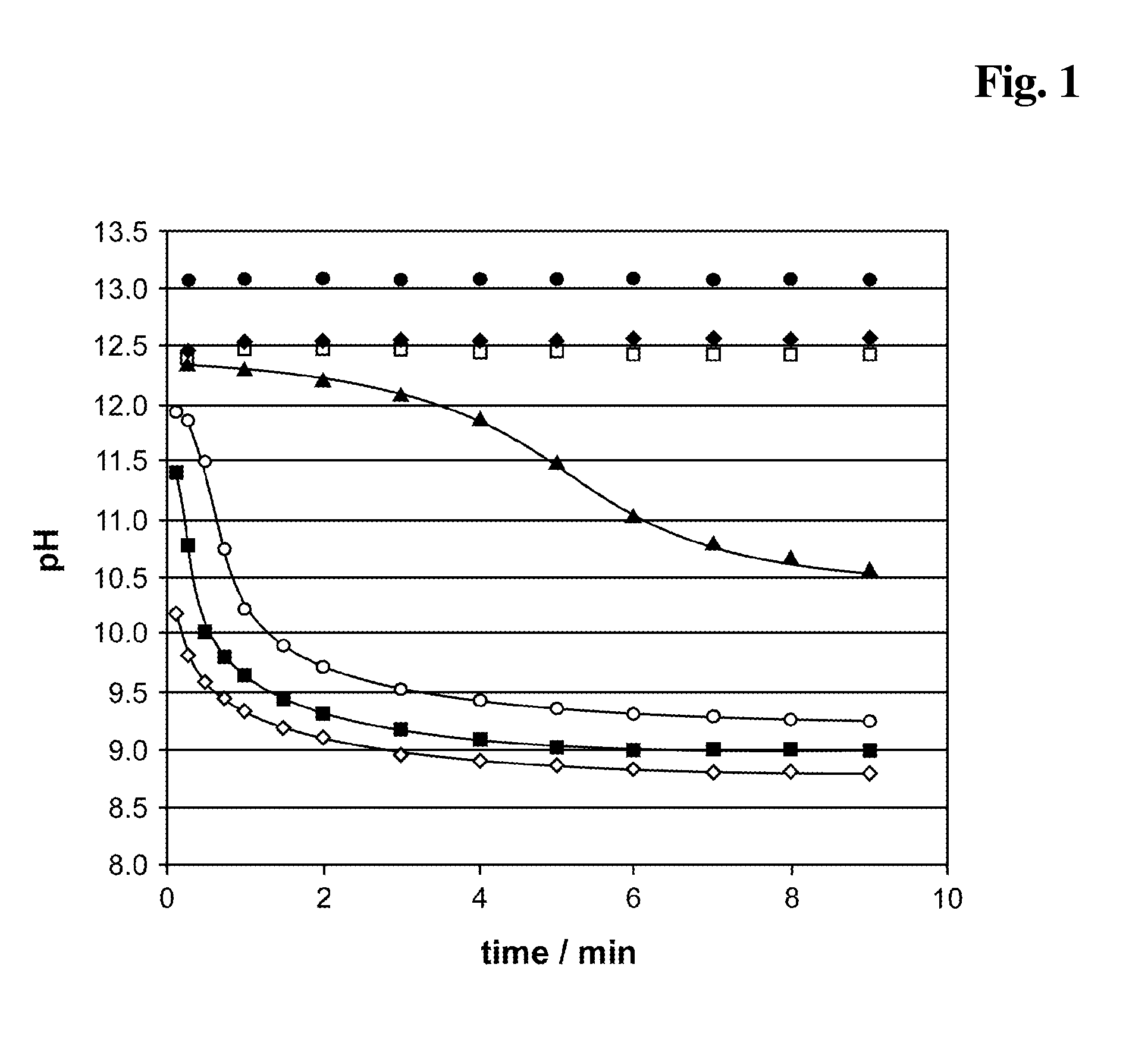
[0206]The sample to be investigated is measured using the Elecsys® system from the Roche Diagnostics company. The total assay procedure is shown in example 1.5. In aberrance to example 1.5 the reagent composition (A) contains either 0.5 M ethylene carbonate (EC), 0.5 M Na2CO3, 0.5 M NaCl or 0.5 M NaH2PO4, respectively.
[0207]Reagent composition (A):
[0208]10 mM NaOH
[0209]4 mM EDTA
[0210]6.7 mM DTT
[0211]0.5 M of either EC, Na2CO3, NaCl or NaH2PO4
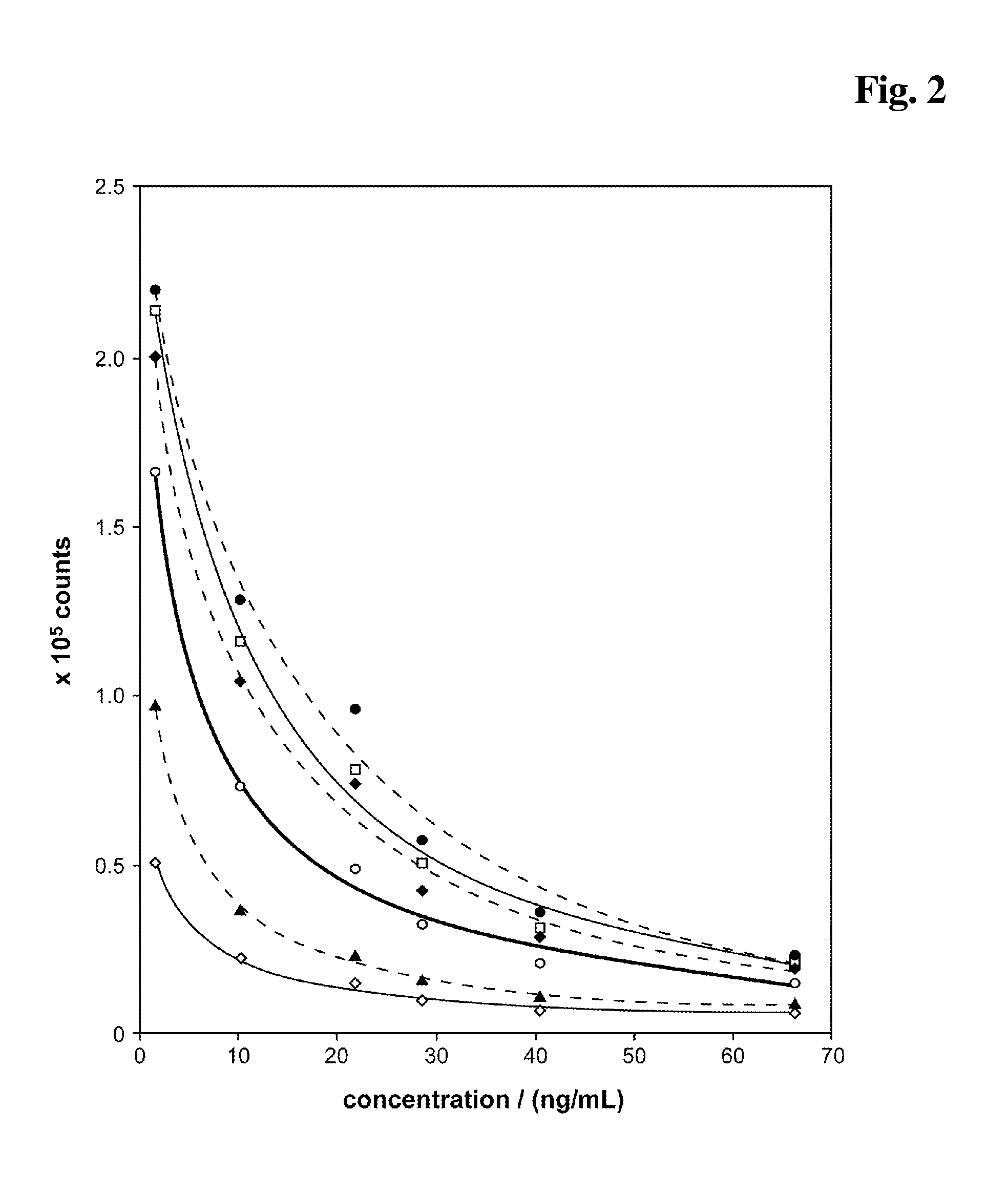
[0212]As control a reagent composition (A) containing 10 mM NaOH, 4 mM EDTA, 6.7 mM DTT has been used. The results are shown in FIG. 5. The carbonate ester (0.5 M EC (♦) present in the alkaline pretreatment (reagent mixture) causes a signal enhancing effect in the competitive assay. Especially the signal dynamic is improved compared to a test without EC (□). A salt (0.5 M NaCl, (⋄)) shows no effect. The addition of 0.5 M Na2CO3 (◯) or 0.5 M NaH2PO4 (▴) shows a mino...
example 3
Alkaline Pretreatment with / without Carbonate Ester
[0213]The sample to be investigated is measured using the Elecsys system from the Roche Diagnostics company. The assay procedure is shown in example 1.5.
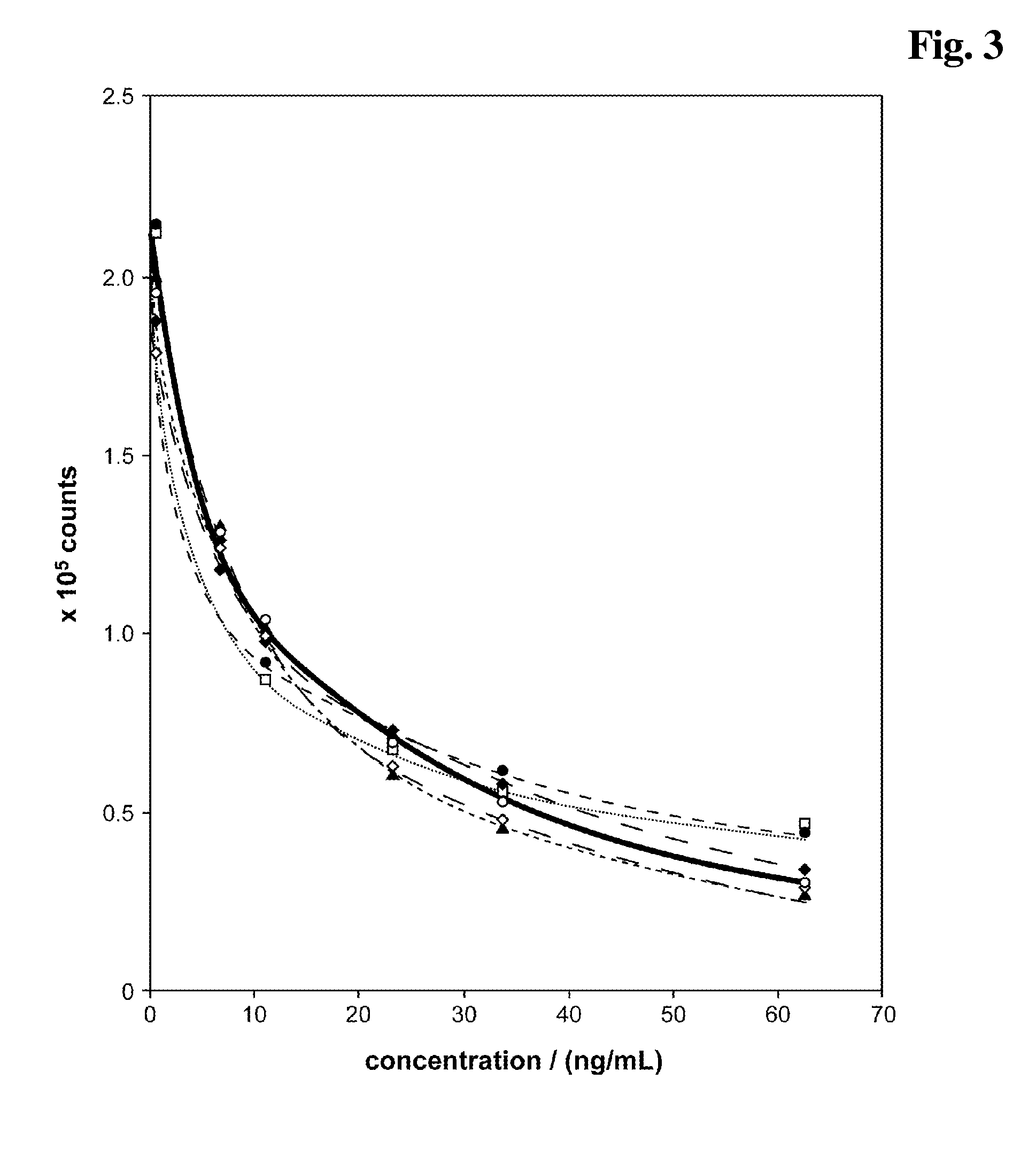
[0214]In aberrance to example 1.5 three different reagent compositions have been prepared containing either:[0215]♦: 10 mM NaOH, 4 mM EDTA, 6.7 mM DTT, 0.5 M EC (see example 1.5) or[0216]▴: 10 mM NaOH, 4 mM EDTA, 6.7 mM DTT or[0217]□:10 mM NaOH, 4 mM EDTA.
[0218]After a 4 min pretreatment incubation of sample+either ♦ (reagent composition (A)+alkalinising agent (B) as described in example 1.5), ▴, or □, respectively, (=reagent mixture) and before addition of solution C the pH of the reagent mixture has been set to pH 9 by addition of bis-tris-propane pH 6.3 (FIG. 6). The carbonate ester EC present in the alkaline pretreatment (reagent mixture) causes a signal enhancing effect in the competitive assay. Especially the signal dynamic is improved compared to a test without EC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com