Colon and pancreas cancer specific antigens and antibodies
a cancer and specific antigen technology, applied in the field of tumor molecular biology, can solve the problems of affecting the patient's health, affecting the detection and treatment of cancer, and affecting the success of cancer diagnosis and treatment, so as to promote tumor regression and slow the growth of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of the NPC-1 Antigen
[0360]The NPC-1 antibody was generated in mice immunized with the so-called “Hollinshead colon cancer vaccine”. Hollinshead, et al. (1985) Cancer 56: 480-489. The NPC-1 antibody and the chimeric form, NPC-1C, are described in U.S. Pat. Nos. 7,314,622 and 7,763,720. Several protein purifications were prepared using both mouse NPC-1 and recombinant, chimeric NPC-1C antibodies. Tumor cell lines including LS174T and HT-29 (human colorectal tumor), CFPAC-1 (human pancreatic tumor), colon cancer patient tumor specimen, and the Hollinshead colon cancer vaccines served as tumor antigen sources for protein extracts. The NPC-1 antigen is secreted into the medium of the human tumor cell lines, and the antigen was purified from tumor cell supernatant of cells grown in the absence of serum for 5 to 7 days. NPC-1 antibody was coupled to resins for the antigen purification, including magnetic beads, for simple adsorption, washing, and elution from the beads. Prot...
example 2
NPC-1 Antigen Knockdown
[0365]A small inhibitory RNA (siRNA) target sequence designed against a region of MUC5AC was used in cells known to express the NPC-1 antigen. Several siRNA oligonucleotides were designed based upon MUC5AC sequences. The sequences of the human MUC5AC siRNA oligonucleotides are shown in Table 7:
TABLE 7Sequence of MUC5AC siRNA oligonucleotidesOligonucleotideStrandSequencesiRNA ID #: s9074SenseAGAUGUGCCUCAACUACGAtt (SEQ ID NO: 120)Anti-SenseUCGUAGUUGAGGCACAUCUtg (SEQ ID NO: 121)siRNA ID #: s9075SenseGCUCUGGAACGUGAGCAUAtt (SEQ ID NO: 122)Anti-SenseUAUGCUCACGUUCCAGAGCcg (SEQ ID NO: 123)siRNA ID #: s9076SenseGCGUGCUCGUCGACAACUAtt (SEQ ID NO: 124)Anti-SenseUAGUUGUCGACGAGCACGCgg (SEQ ID NO: 125)
[0366]The siRNAs were transfected into LS174 and CFPAC-1 tumor cells, as well as A549 cells. A549 is a human lung adenocarcinoma cell line that expresses MUC5AC (as shown by PCR and detection using commercially available antibodies against MUC5AC), but not NPC-1 antigen. The MU...
example 3
NPC-1 Epitope Mapping
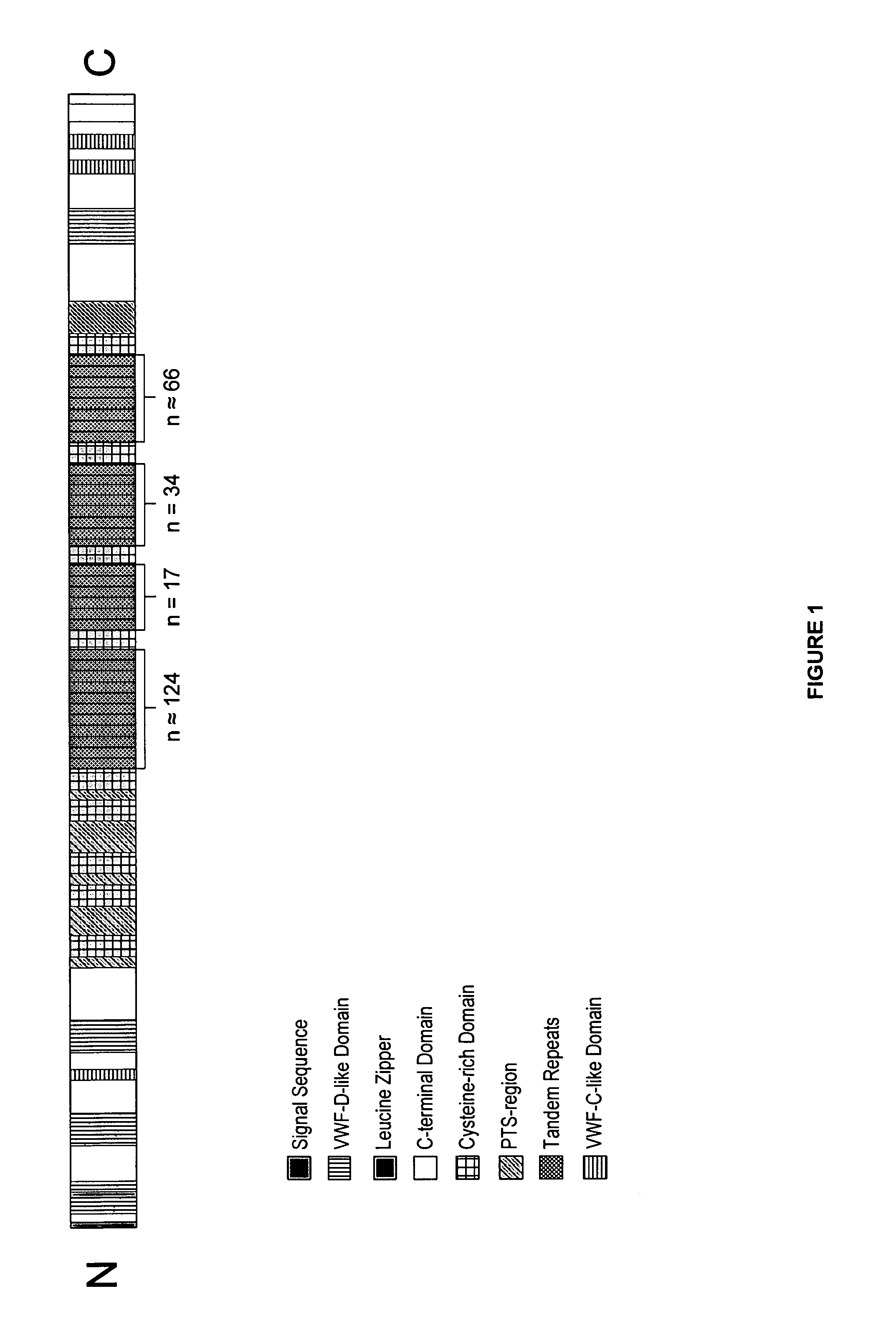
[0368]The data by western blots of trypsin-digested MUC5AC indicated that there may be several NPC-1 antibody binding sites on each molecule of MUC5AC, suggesting that a binding region might be present in each of the tandem repeat units located in the central region of the molecule. Therefore, a recombinant expression plasmid was designed and constructed that encoded residues upstream of tandem repeat units and two tandem repeat units (“MUC5AC-long” SEQ ID NO: 3). The MUC5AC long peptide corresponds to amino acid residues 2636 to 2942 of the MUC5AC protein (SEQ ID NO: 1). A second expression plasmid was designed and constructed that encoded primarily a short domain which connects to the central repetitive region and only a portion of the tandem repeat residues (“MUC5AC-short” SEQ ID NO: 5). The MUC5AC short peptide corresponds to amino acid residues 2636 to 2763 of the MUC5AC protein (SEQ ID NO: 1 and FIG. 1). These smaller fragments of the large MUC5AC molecule...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com