Use of akt phosphorylation as a biomarker for prognosing neurodegenerative diseases and treating same
a neurodegenerative disease and biomarker technology, applied in the field of neurodegenerative diseases treatment with akt activating agents, can solve the problems of inability to teach or even suggest the use of pakt, paralysis and muscle atrophy, and als is a devastating and rapidly fatal disease, so as to prolong the life span of said mice, prolong the survival, and enhance the effect of survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effect of the Peptide of SEQ ID NO: 1 on the Akt Pathway in Cells
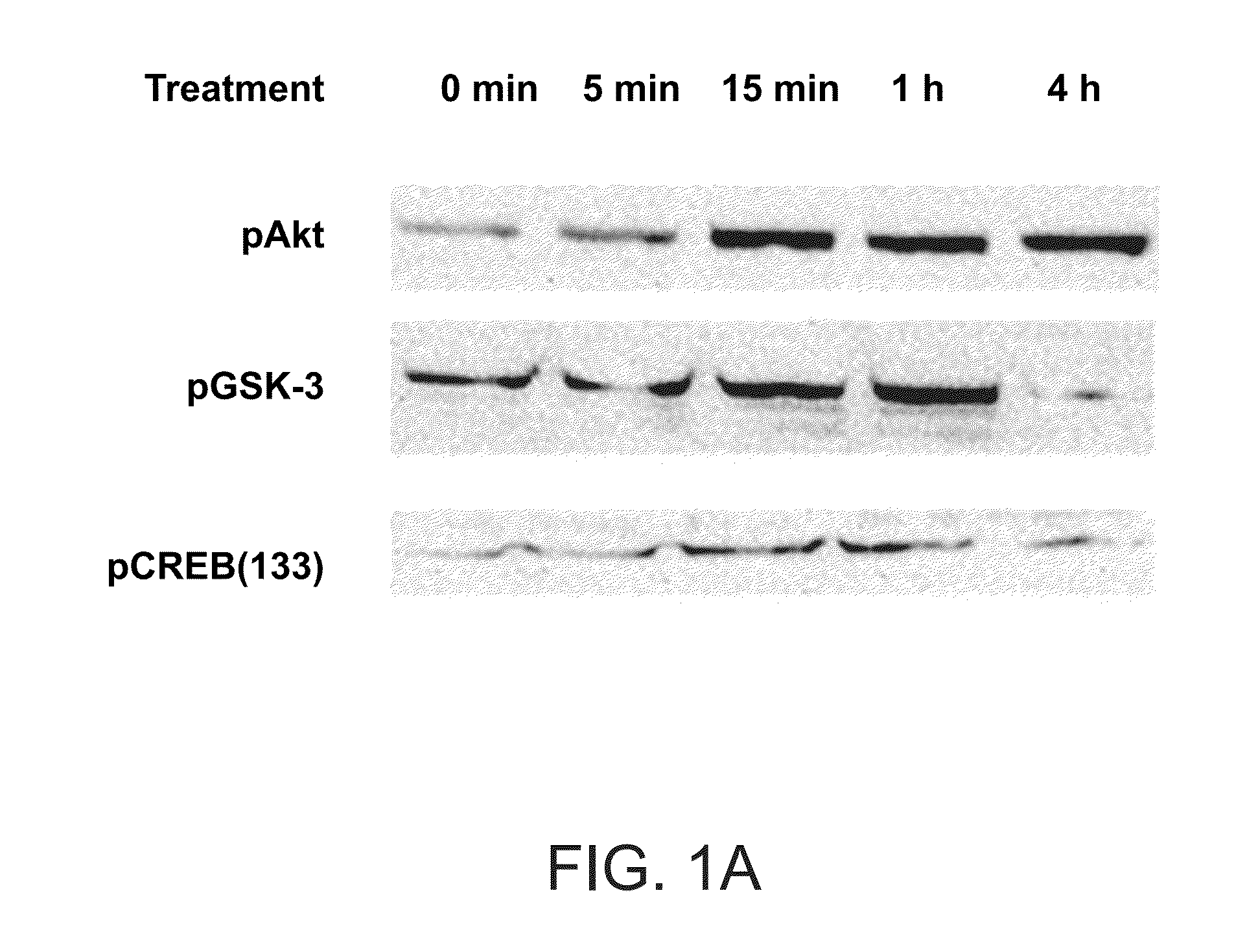
[0186]Raw264.7 macrophages were treated with 50 mM of the peptide having the amino acid sequence of SEQ ID NO: 1 (10×5 per well) in quadruplicates. Akt phosphorylation was found to be induced shortly after introduction of the peptide (FIG. 1A). Akt and pAkt were detected using western blot analysis with Cell Signaling Technology #9272 and #9275 antibodies. This observation demonstrates that the peptide triggers activation of the Akt pathway in a specific manner.
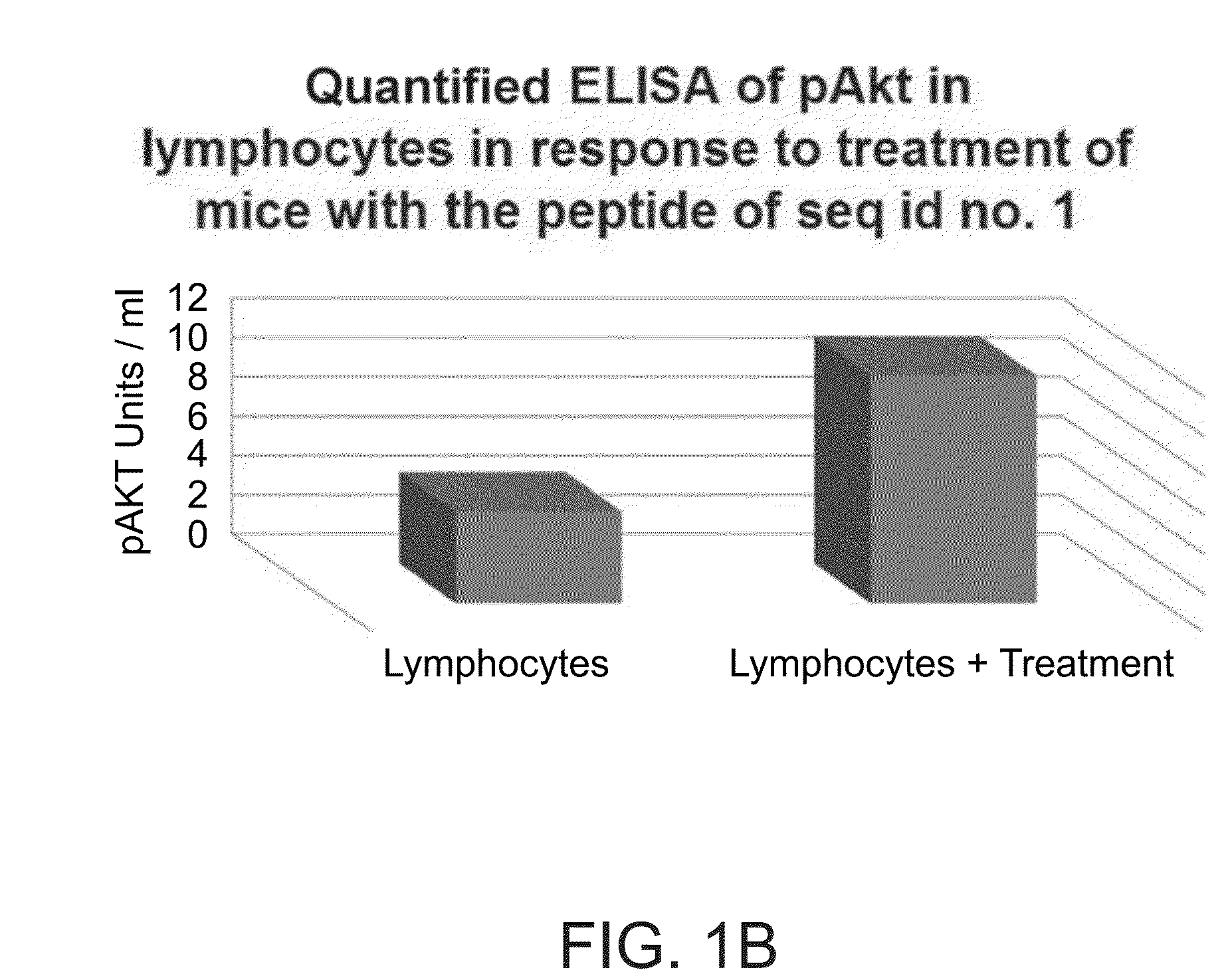
[0187]Next, mice were injected with the peptide of SEQ ID NO: 1 (400 μl) and after 30 minutes lymph nodes were assessed for pAkt, compared to lymph samples obtained from non-treated mice. Akt phosphorylation was measured using ELISA compared to a standard, using Millipore's STAR Akt and STAR phospho-Akt1 (Thr308) ELISA kits.
[0188]As shown in FIG. 1B, Akt phosphorylation was higher in treated mice compared to non-treated mice.
example 2
The Effect of the Peptide of SEQ ID NO: 1 on Survival of a Mice Model for ALS
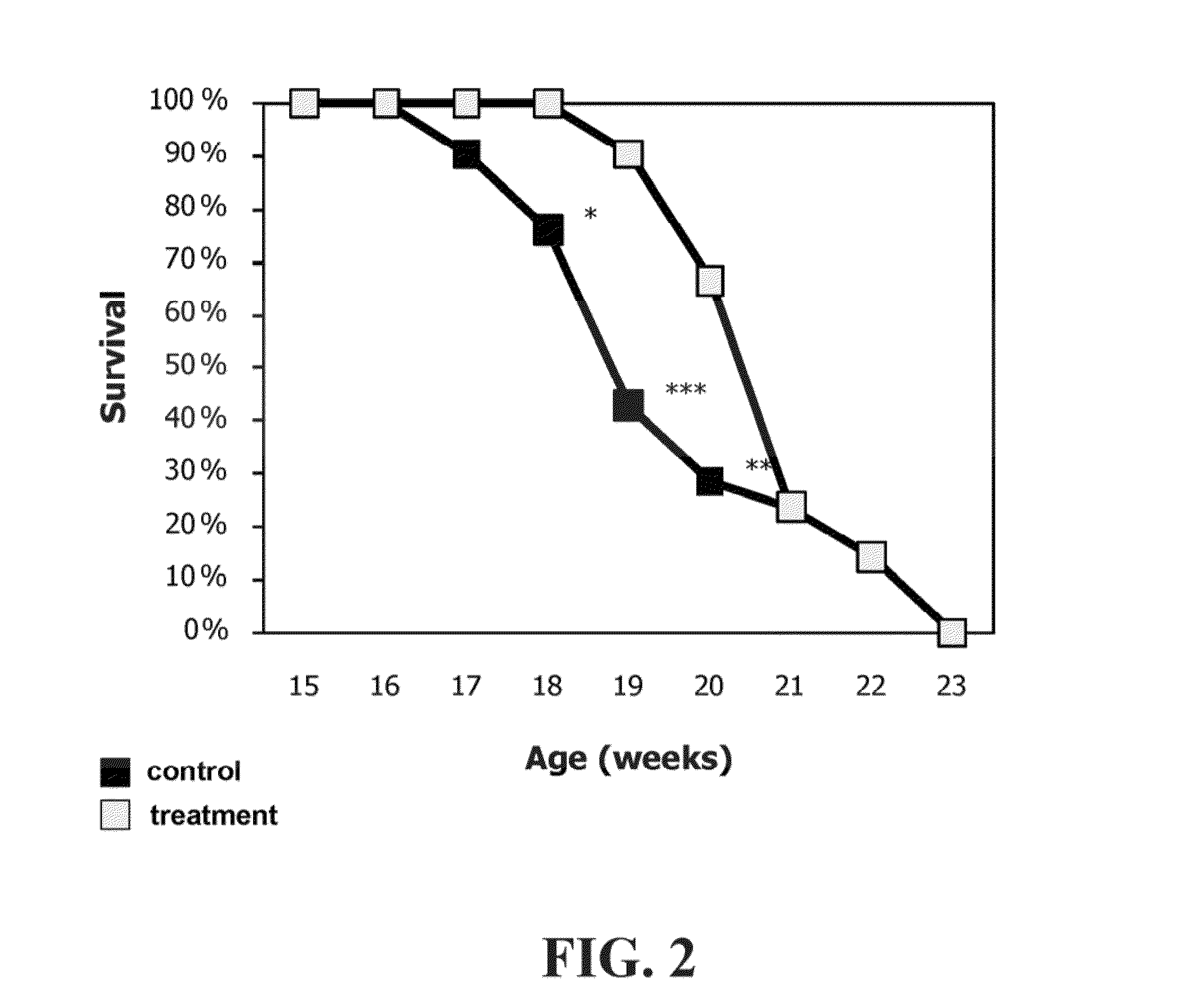
[0189]ALS is characterized by apoptosis of motor neurons. The SOD1G93A mouse model of familial ALS was used in the following examples. Initially, doses less than optimal were applied, and model irregularities due to gene counts were detected. These deficiencies were repaired in later studies.
[0190]Mice were purchased from Jackson and were administered with a dose of at least 200 microgram a day of the peptide of SEQ ID NO: 1. Overall, the studies included 142 mice. Eventually all mice died of neurologic disease. Mice (a few) that died before the detection of a symptomatic disease were not included, as recommended by Scott et al. (Amyotroph Lateral Scler. 2008; 9(1):4-15). Of the 142 mice, 99 mice were treated intraperitoneally (IP), intranasally (IN), intravenously (IV) or with a combined delivery, and 43 mice were used as control (untreated or treated with PBS).
[0191]Allocation of the mice to the experimen...
example 3
The Effect of the Peptide of the Invention on Disease Progression
[0200]The protective effect of the peptide of the invention is not only limited to prolonged survival, but also involves significant delay in the progression of disease symptoms, as monitored by the neurologic disability score, thereby postponing development of end-stage disease.
[0201]Mice were administered with the peptide of SEQ ID NO: 1 or placebo (PBS) starting at disease onset as measured by weight loss. It was at average age of about 90 days. The mice were treated daily. During the period of weeks 16-19, the neurologic score was assessed, and was found lower by a factor of about 2 in treated mice compared to controls (FIG. 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ratio | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com