RANKL ANTIBODY-PTH/PTHrP CHIMERIC MOLECULES
a technology of pthrp and pthrp, which is applied in the field of pthrp/pthrp chimeric molecules, and can solve problems such as bone density increas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparing synPTH-αRANKL-1 Light Chain Expression Plasmid
[0450]A synthetic oligonucleotide having the sequence shown in FIG. 7 (SEQ ID NO: 5) was obtained from Picoscript (Houston, Tex.). That oligonucleotide sequence contains a 5′ XbaI restriction site (TCTAGA) followed by a Kozak sequence (CCACC), which are shown in bold in FIG. 7. The oligonucleotide sequence also contains a synthetic coding sequence for the first 65 amino acids of human preproparathyroid protein (prepropTH). See, e.g., Genbank accession no. CAA23843. The first 65 amino acids of human prepropTH contains a prepro domain and amino acids 1-34 of the human PTH modulating domain. The oligonucleotide sequence also contains a coding sequence for a helical linker sequence, GGGAP. That linker coding sequence also contains a BSSHII restriction site (GCGCGC). The synthetic oligonucleotide was cloned into plasmid pCR4.0-TOPO by Picoscript prior to delivery (oligo-pCR4.0 TOPO). Oligo-pCR4.0 TOPO was digested with XbaI and BssH...
example 2
Preparing synPTH-αRANKL-1 Heavy Chain Expression Plasmid
[0455]The synPTH coding sequence was prepared as described above in Example 1.
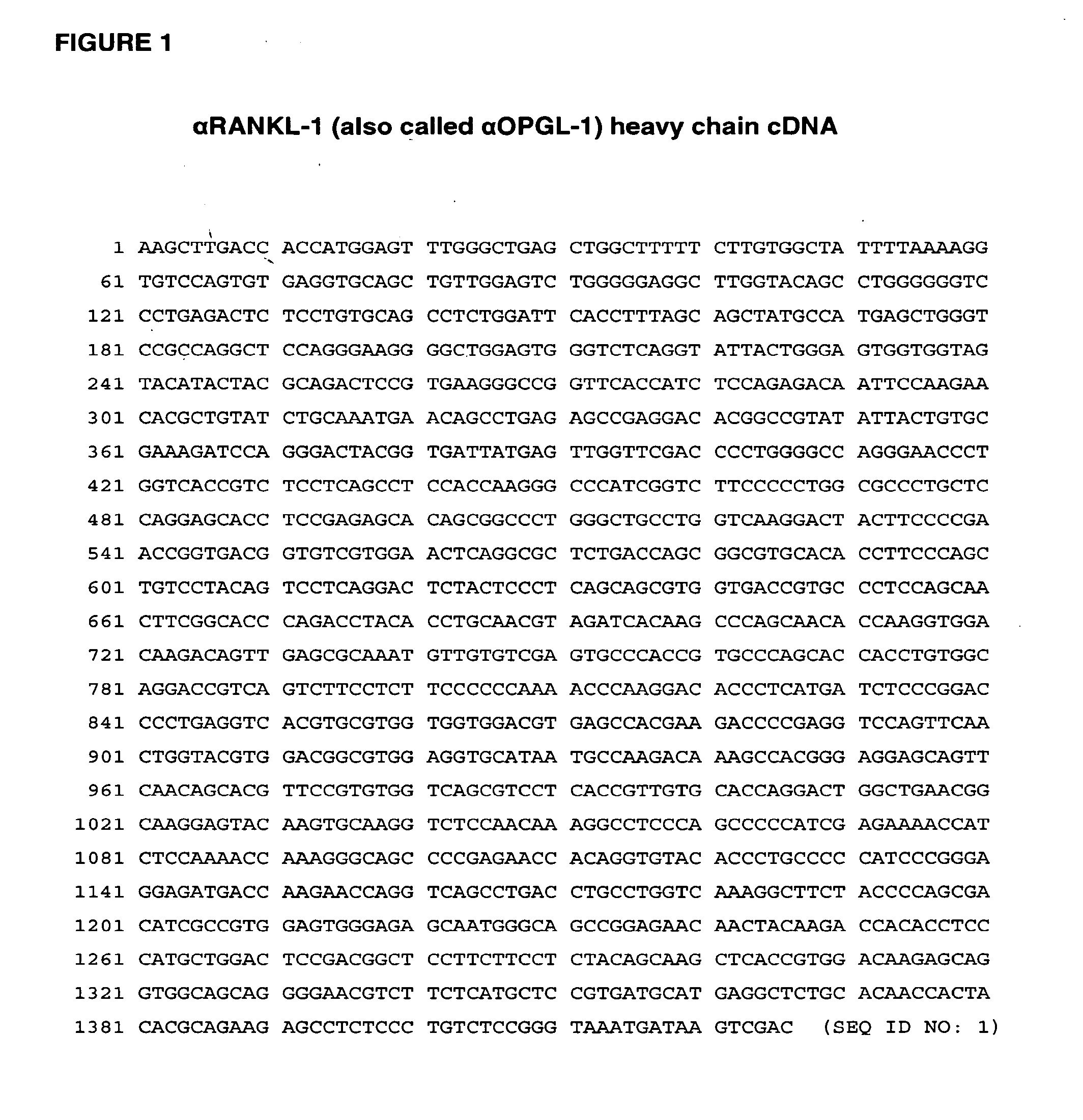
[0456]The αRANKL-1 (also called αOPGL-1) heavy chain was amplified from αRANKL-1-IgG2 / pDSRα19 plasmid (also called αOPGL-1-IgG2 / pDSRα19, described in PCT Publication No. WO 03 / 002713) as follows. Ten ng of αRANKL-1-IgG2 / pDSRα19 plasmid DNA was used in a PCR reaction using Pfu polymerase (Stratagene). The following primers were included in the reaction:
5′αRANKL-1 IgG2 BssHII primer (SEQ ID NO: 216): BssRII G A P E V Q L L E5′-AA CTT GGC GCG CCC GAG GTG CAG CTG TTG GAG-3′3′ human IgG2 constant region primer(SEQ ID NO: 217):5′-G CAT GTC GAC TCA TTT ACC CGG AGA CAG GGA SalI * K G P S L S GAG-3′ L
[0457]The PCR reaction generated a 1372 base pair PCR product, which encodes the amino acid sequence of αRANKL-1 heavy chain with 3 amino acids (GAP) of the linker sequence on the N-terminus. The 1372 ba...
example 3
Expression of synPTH-αRANKL-1
Expression in Chinese Hamster Ovary (CHO) Cells
[0460]For expression of synPTH-αRANKL-1 heavy chain fusion, dihydrofolate reductase deficient (DHFR-) serum-free adapted CHO AM-1 / D (described in U.S. Pat. No. 6,210,924) cells were co-transfected with synPTH-αRANKL-1-IgG2 pDSRα20 and αRANKL-1-kappa / pDSRα19 (also called αOPGL-1-kappa / pDSRα19; see PCT Publication No. WO 03 / 002713) using the calcium phosphate method. For expression of synPTH-αRANKL-1 light chain fusion, dihydrofolate reductase deficient (DHFR-) serum-free adapted CHO AM-1 / D cells were co-transfected with synPTH-αRANKL-1-kappa pDSRα20 and αRANKL-1-IgG2 / pDSRα19 (also called αOPGL-1-IgG2 / pDSRα19; see PCT Publication No. WO 03 / 002713) using the calcium phosphate method.
[0461]Expression of each of the synPTH-αRANKL-1 heavy chain fusion and the synPTH-αRANKL-1 light chain fusion was carried out as follows. Transfected cells were plated in 10 cm plates and selected in DMEM media supplemented with 1× ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com