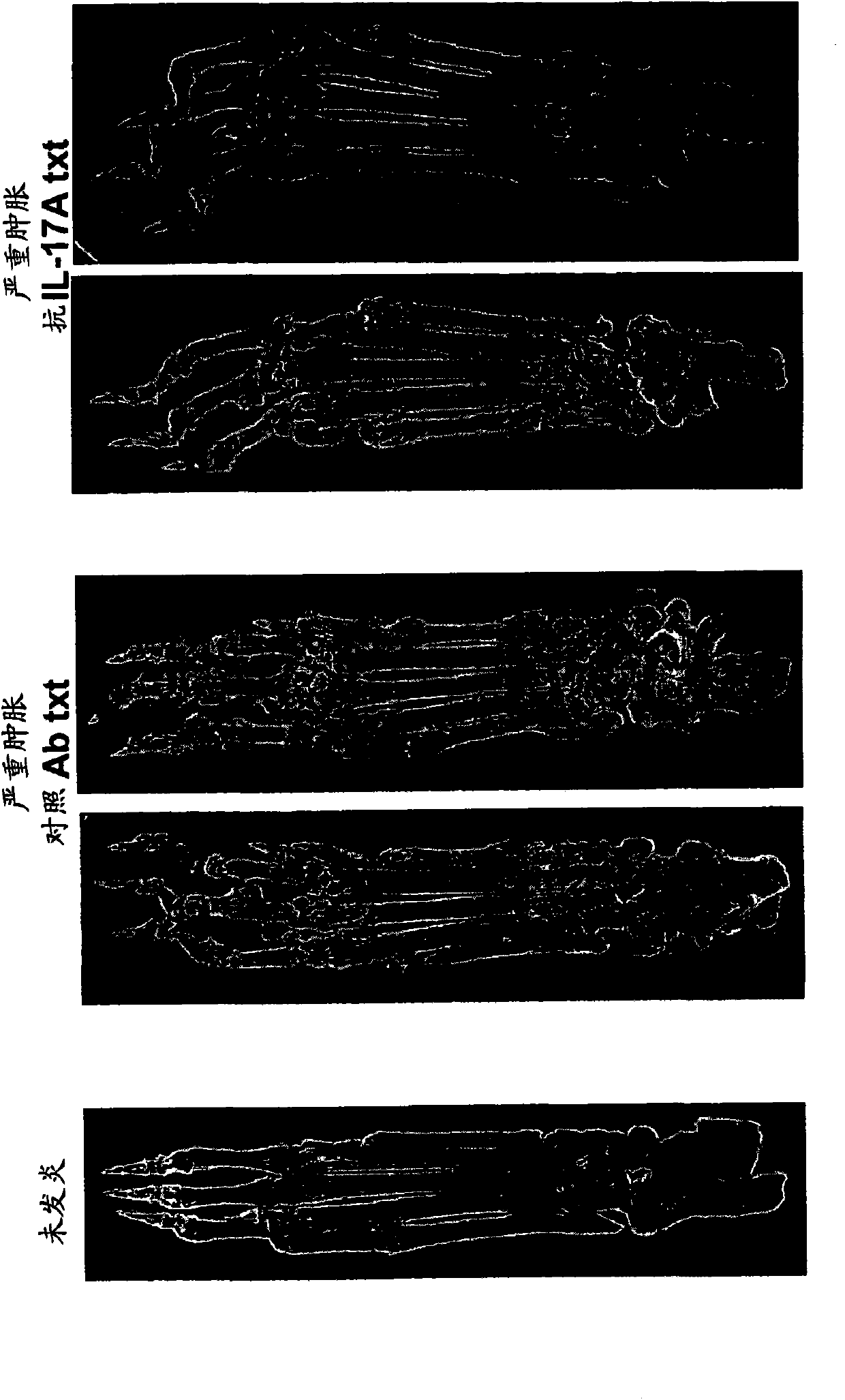
Joint destruction biomarkers for anti-il-17a therapy of inflammatory joint disease
A biomarker, inflammatory joint technology, applied in biological testing, biological material analysis, antibodies, etc., can solve problems such as not seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0120] Antibody antagonists for use in the present invention can be prepared by any method known in the art for preparing antibodies. Preparation of monoclonal antibodies, polyclonal antibodies and humanized antibodies can be found in Sheperd and Dean (eds.) (2000) Monoclonal Antibodies, Oxford Univ. Press, New York, NY; Kontermann and Dubel (eds.) (2001) Antibody Engineering, Springer -Verlag, New York; Harlow and Lane (1988) Antibodies A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 139-243; Carpenter et al. (2000) J. Immunol. 165:6205; He et al (1998) J. Immunol. 160: 1029; Tang et al. (1999) J. Biol. Chem. 274: 27371-27378; Baca et al. (1997) J. Biol. Chem. 272: 10678-10684; Chothia et al. Nature 342:877-883; Foote and Winter (1992) J. Mol. Biol. 224:487-499; and US Patent No. 6,329,511 (to Vasquez et al.).
[0121] Any antigenic form of the desired target can be used to generate antibodies against which antibodies can be screened for...
Embodiment 1
[0145] NHDF assay for anti-IL-17A antibody
[0146] Blocking of IL-17A biological activity by anti-IL-17A antibodies used in the invention was determined by monitoring rhIL-17A-induced IL-6 expression in a normal human (adult) dermal fibroblast (NHDF) primary cell line ability. Briefly, different concentrations of anti-IL-17A antibodies to be tested were incubated with rhIL-17A, and the resulting mixture was added to NHDF cell cultures. IL-6 production was then assayed as a measure of the antibody's ability to inhibit IL-17A activity. A more detailed scheme is as follows.
[0147]A series of 2-fold dilutions of the anti-IL-17A antibody of interest (in duplicate) was prepared starting with a 40 μg / ml stock solution. A 120 ng / ml rhIL-17A stock solution was prepared. 70 μl rhIL-17A stock solution was mixed with 70 μl anti-IL-17A antibody dilution in wells of a microtiter plate and incubated at room temperature for 20 minutes. 100 μl of each of these mixtures was then added t...
Embodiment 2
[0151] Anti-IL-17A antibody assay in foreskin fibroblasts
[0152] The ability of the anti-IL-17A antibodies used in the present invention to block the biological activity of IL-17A was determined by monitoring the rhIL-17A-induced IL-6 expression in the HS68 foreskin fibroblast cell line. Reduced IL-6 production in response to rhIL-17A can be used as a measure of the blocking activity of the anti-IL-17A antibodies used in the invention.
[0153] IL-17RC (IL-17A receptor) expression was analyzed in a panel of fibroblast cell lines identified as potential IL-17A responsive cell lines in the human foreskin fibroblast cell line HS68 (ATCC CRL1635). This was confirmed by the following experiments: by using polyclonal goat anti-human IL-17R antibody (R&D Systems, Gaithersburg, Maryland, USA), followed by phycoerythrin (PE)-F(ab') 2 Indirect immunofluorescence staining of donkey anti-goat IgG (Jackson Immunoresearch, Inc., WestGrove, Pennsylvania, USA) and analysis of PE immunofluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com