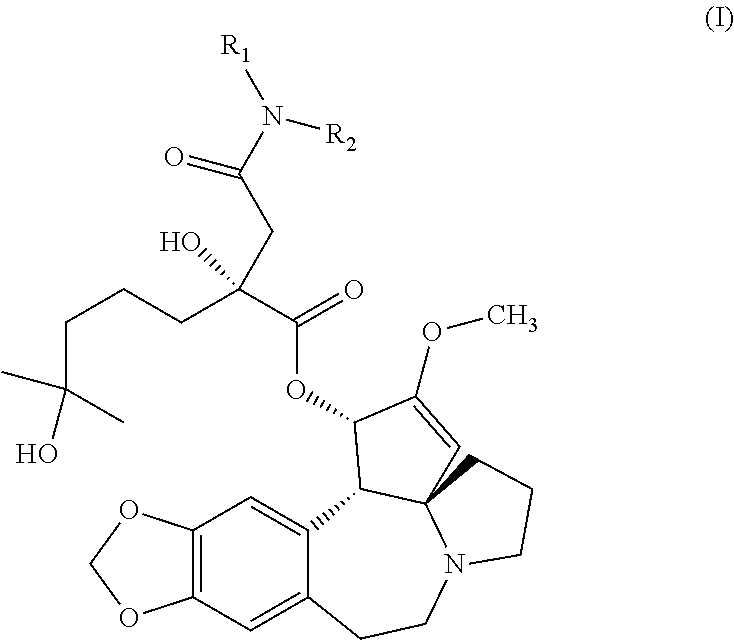
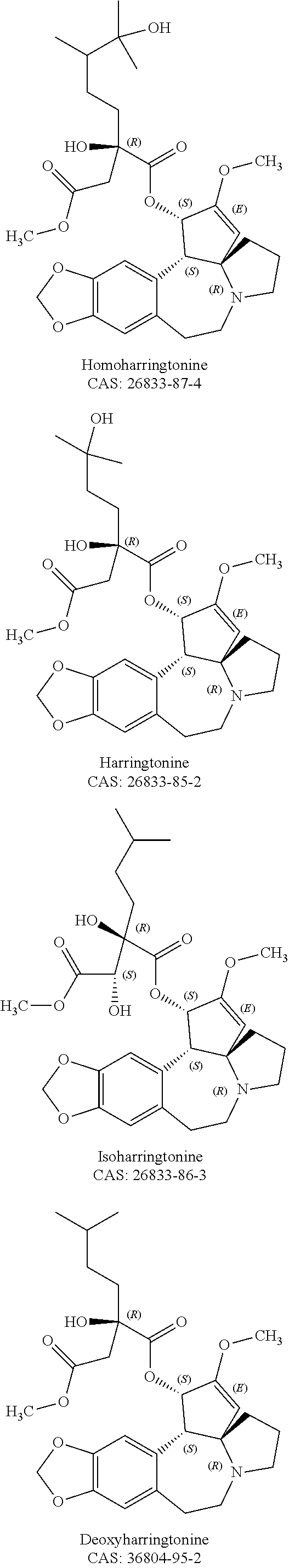
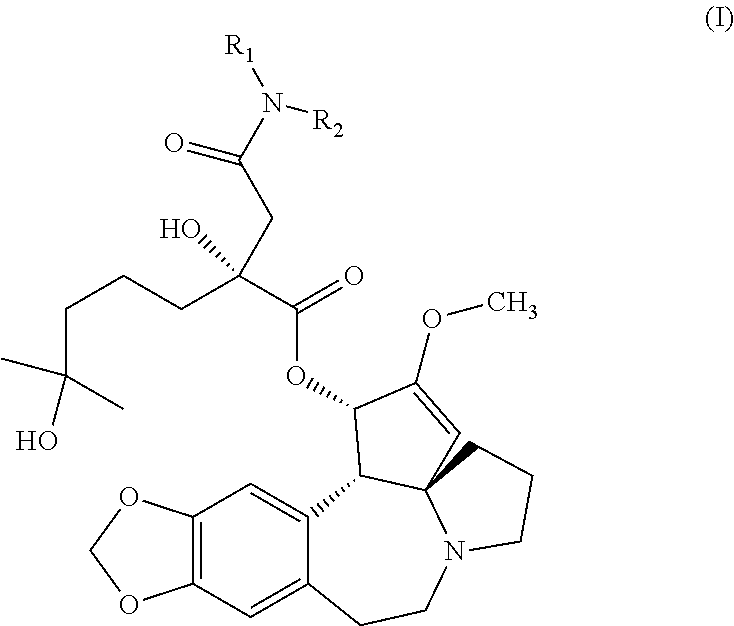
Aminated derivative of homoharringtonine, preparation method therefor, and application thereof
a technology homoharringtonine derivatives, which is applied in the field of natural medicine and pharmaceutical chemistry, can solve the problems of unreported synthesis and application of aminated or amidated homoharringtonine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Synthesis of Compound BS-HH-043
[0091]
wherein, X01-1: homoharringtonine acid; Zi: furan-2-ethylamine; HATU: 2-(7-azobenzotriazolyl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate; DIPEA: N,N-diisopropylethylamine.
[0092]Homoharringtonine is hydrolyzed according to the general scheme above. Afterwards, the acid resulted from the hydrolysis of homoharringtonine, as an intermediate, X01-1(106 mg, 0.2 mmol), and 2-aminomethylfuran (24 mg, 0.24 mmol) are dissolved in anhydrous DMF (2 mL). N,N-diisopropylethylamine (52 mg, 0.4 mmol) and 2-(7-azobenzotriazolyl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (114 mg, 0.3 mmol) are added and the mixture is stirred for 3 h under 30° C. Water (6 mL) is added under 5-10° C. and the mixture is extracted with ethyl acetate. The organic phase is washed with a saturated saline solution, dried and concentrated. The crude product is separated and purified with a silicagel column (DCM: methanol=10:1) to give BS-HH-043 (9 mg, 6%) as a colorless o...
example 2
Evaluation of the Aminated Homoharringtonine Derivatives of the Present Invention for Their Anti-Leukemia Activities
[0158](1) Experimental Materials
[0159]Leukemia cell lines: K562 / adr (drug-resistant, chronic myeloid leukemia, CML), NB4 (acute promyelocytic leukemia, AML), Kasumi-1 (acute myeloid leukemia M2 type, AML-M2), Jurkat (acute lymphoblastic leukemia, ALL), all of which are donated by Cancer Research Institute of Zhejiang University, China; and H9 (acute lymphoblastic leukemia, ALL), which is purchased from China Center for Type Culture Collection.
[0160]Reagents: The standard sample of homoharringtonine (HHT) is purchased from Taihua Natural Plant Pharmaceutical Co., Ltd., Shaanxi, China; and the homoharringtonine derivatives of the present invention.
[0161]Main apparatuses: a Thermo Scientific 3111 incubator and a Bio-Rad iMark microplate reader.
[0162](2) Experimental Method
[0163]Obtaining 6000 well-growing leukemia cells and inoculating them into wells of a 96-well cell cu...
example 3
Evaluation of the Aminated Homoharringtonine Derivatives of the Present Invention for Their Anti-Human Multiple Myeloma and Lymphoma Cell Activities
[0166](1) Experimental Materials
[0167]Multiple myeloma and lymphoma cell lines: RPMI8226 (multiple myeloma), purchased from Fuxiang Bio-tech Co. Ltd., Shanghai, China.
[0168]Reagents: the same as in Example 2.
[0169]Main apparatuses: a Thermo Scientific 3111 incubator and a Bio-Rad iMark microplate reader.
[0170](2) Experimental Method
[0171]Obtaining 6000 well-growing leukemia cells and inoculating them into wells of a 96-well cell culture plate. The culture medium is the 1640 cell culture medium containing 10% fetal bovine serum. After adding the homoharringtonine derivatives of different concentrations and mixing uniformly, placing the plate in a carbon dioxide cell incubator (5% CO2) at 37° C. and incubating for 72 hours. Then the viable cell concentration is determined by the MTT method. In this experiment, the cell viability in control...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| organic | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com