Incorporation of methyl lysine into polypeptides
a technology of methyl lysine and polypeptides, which is applied in the field of genetically encoding nemethylllysine in recombinant polypeptides, can solve the problems of unpredictable effects on the properties of analogs, difficult comparisons between analogs and natural modifications, and often challenging experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Polypeptide Comprising Nε-Methyl-Lysine
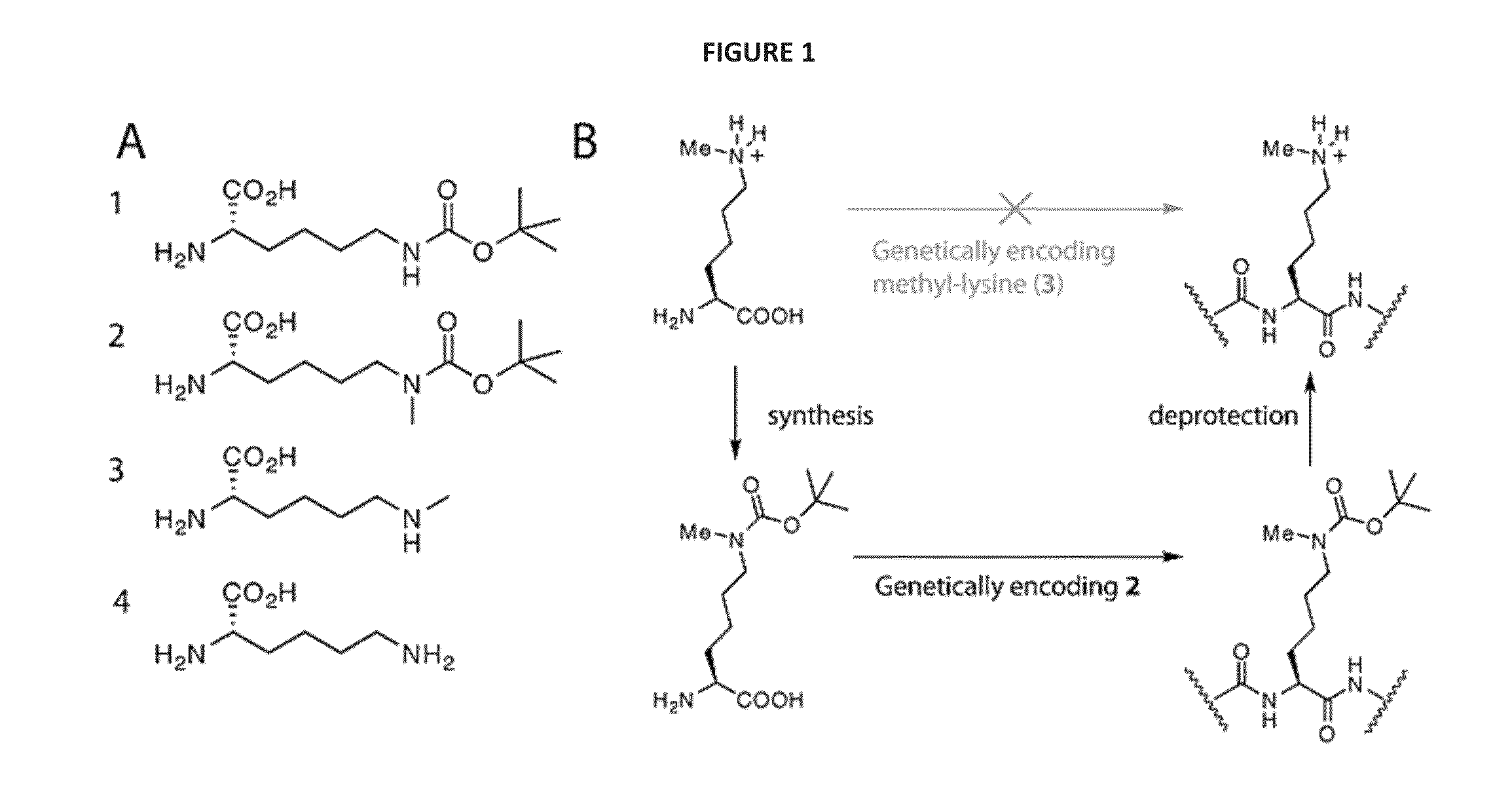
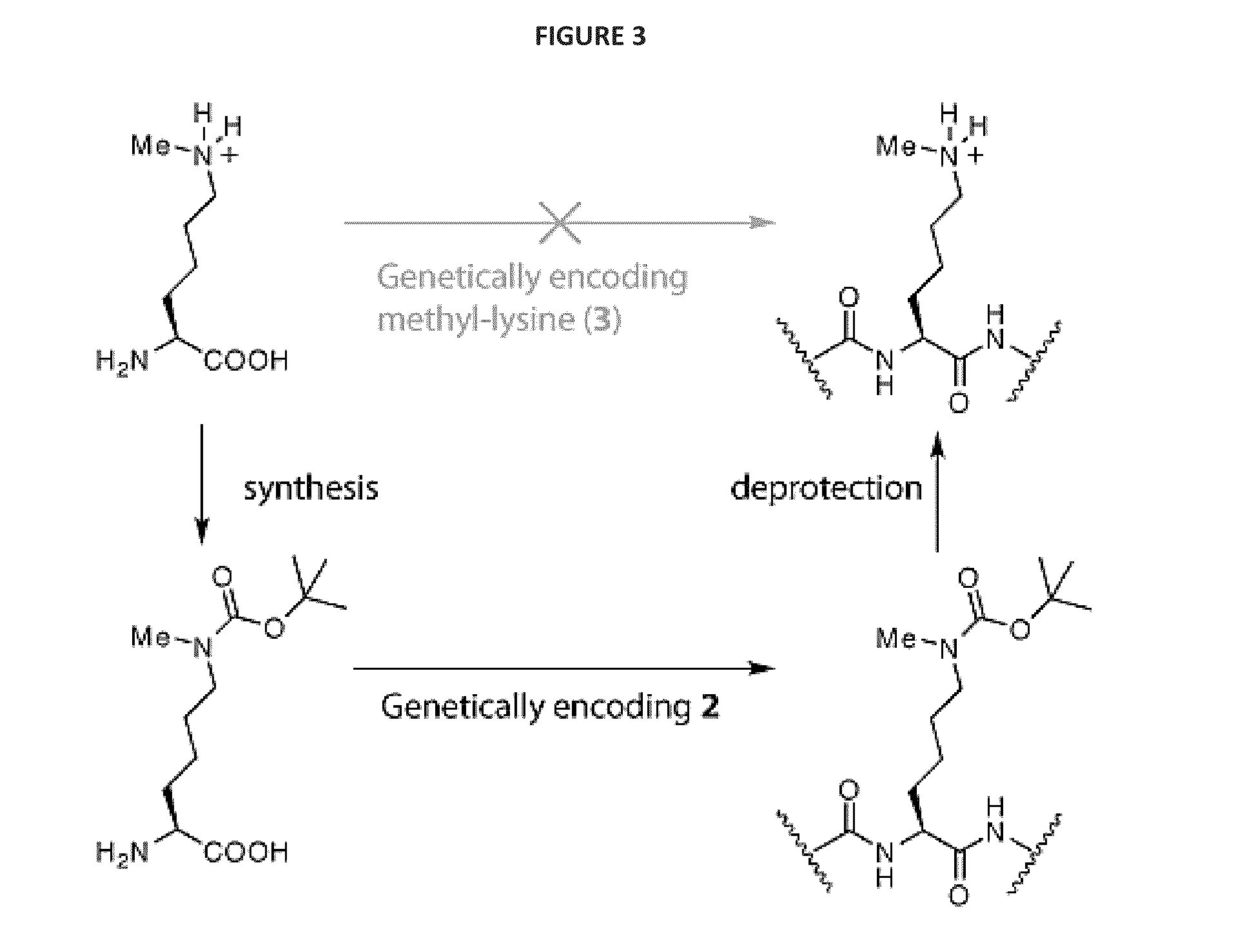
[0109]We realized that we might be able to encode Nε-methyl-L-lysine (3) indirectly by providing the synthetase enzyme with a substrate that was significantly different from both Nε-methyl-L-lysine and L-lysine if we were able to subsequently effect the facile, quantitative and specific post-translational conversion of this precursor to Nε-methyl-L-lysine on the synthesized protein. Since Nε-tert-butyl-oxycarbonyl-L-lysine (1) is an efficient substrate for the pyrrolysyl-tRNA synthetase / tRNACUA pair19 we asked whether Nε-methyl-L-lysine (3) could be incorporated into proteins in a two-step process in which Nε-tert-butyl-oxycarbonyl-Nε-methyl-L-lysine (2) is genetically incorporated into proteins and the tert-butyl-oxycarbonyl group is removed post-translationally to reveal Nε-methyl-L-lysine (see FIG. 1—Strategies for encoding lysine methylation. A. amino acids used B. Schemes for encoding 3 in recombinant proteins.)
[0110]To inves...
example 2
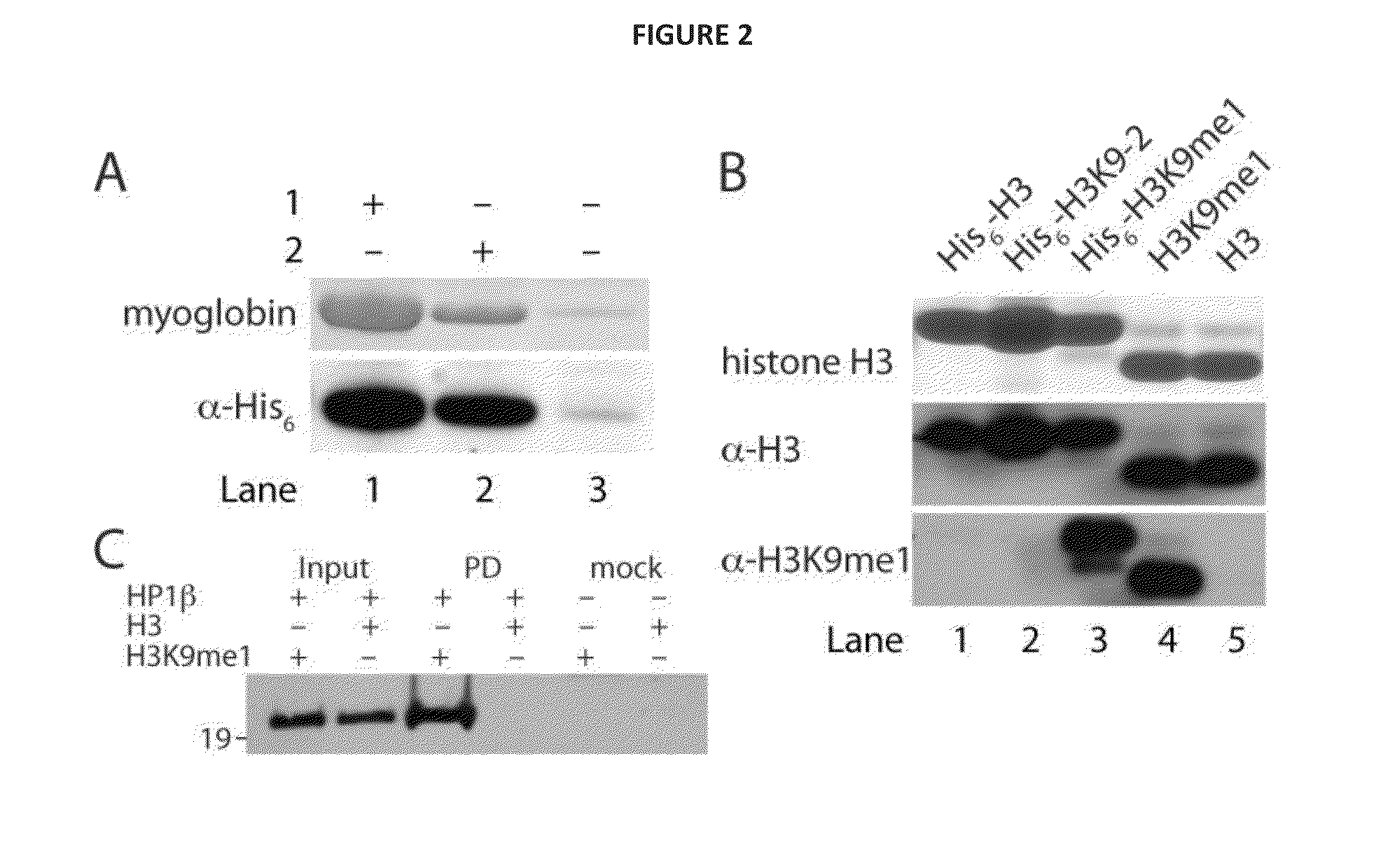
[0111]To demonstrate that 2 can be incorporated with high fidelity into recombinant proteins and is not subjected to in vivo modification14, we performed electrospray ionization mass spectrometry (ESI-MS) on the purified protein. The ESI-MS spectra of myoglobin-His6 demonstrates the quantitative incorporation of 2 (FIG. 7A). These data demonstrate that 2 can be genetically encoded in proteins in good yield and with high fidelity using MbPylRS / MbtRNACUA pair.
example 3
Application to Histones
[0112]To specifically and efficiently introduce 2 in a histone at physiologically relevant site, we transformed E. coli BL21(DE3) with pBKPylS and pCDF-PylT-H3K9TAG (a vector which encodes MbtRNACUA and a N-terminally hexahistidine tagged histone H3 gene in which the codon for lysine 9 is replaced with an amber codon)15. We grew the cells in the presence of 2 mM 2, and expressed and purified the recombinant histone in good yield (2 mg per liter of culture). ESI-MS analysis of the purified histone confirms the incorporation of 2 into histone H3 (FIG. 7B).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com