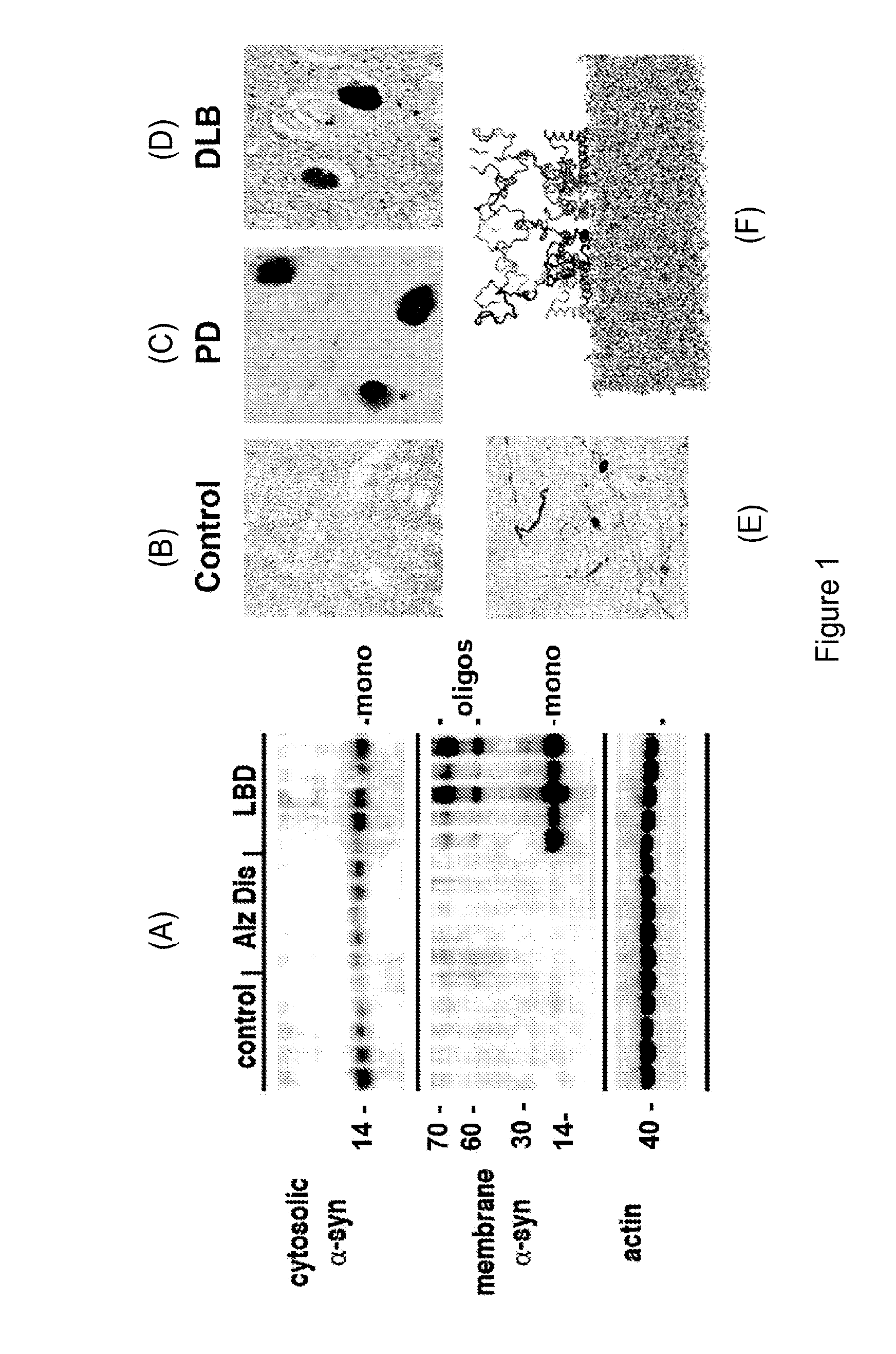
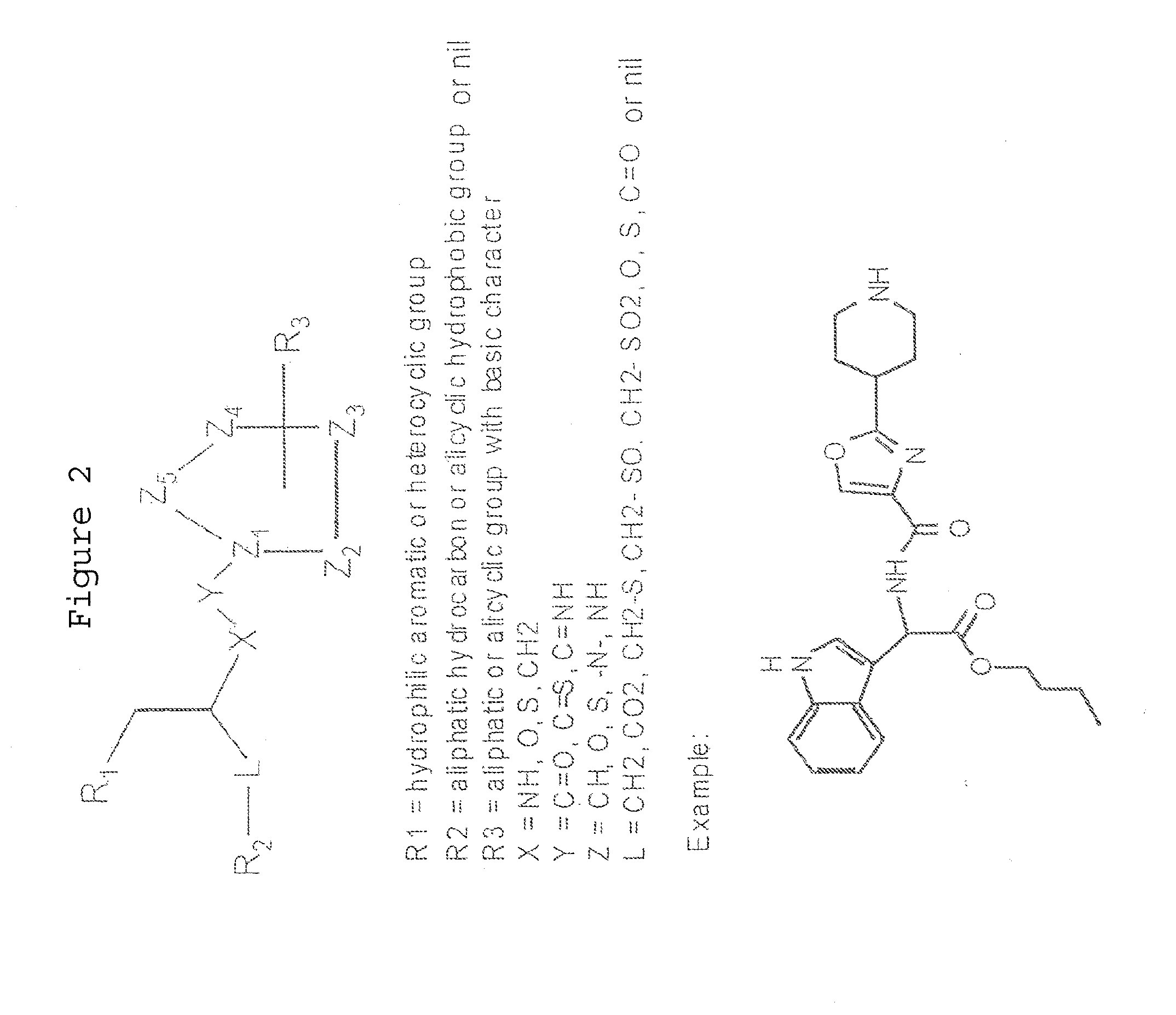
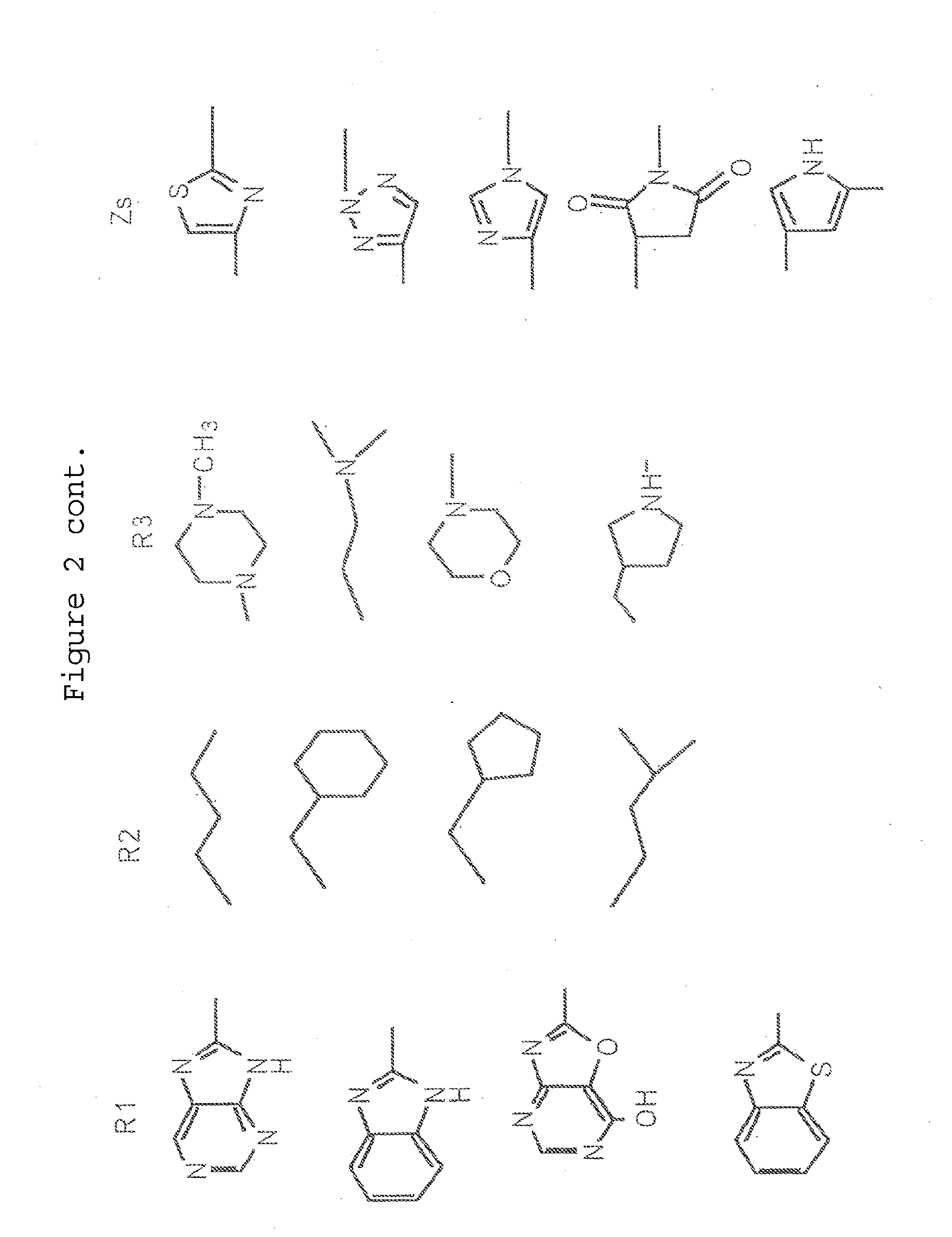
Compound suitable for the treatment of synucleopathies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0085]The chemical synthesis of the compounds having formula (I) as defined above is exemplified by the synthesis of the compound having the following structure:
[0086]The synthesis scheme is depicted in FIG. 8.
1. Synthesis of 2-piperazinyl-thiazole-5-carboxylic acid 2
[0087]2-Bromothiazole carboxylic acid 1 (1 g; 4.8 mmol) and piperazine (6.2 g; 72 mmol; 24 equiv.) were dissolved in dioxane, K2CO3 (3.32 g; 24 mmol; 5 equiv.) was added and the suspension was refluxed overnight. The solvent was removed at the rotary evaporator, the residue dissolved in ethanol, filtered and recrystallized. The crude product was dried at the oil pump and directly used in the following step.
2. Protection of 2 with Boc
[0088]Crude 1, triethylamine (24 mmol; 3.4 ml) and di-tert-butyl-dicarbonate (14.4 mmol; 3.14 g) were dissolved in methanol and refluxed overnight. The solvents were removed at the rotary evaporator and the residue was chromatographed over a short silica gel column.
[0089]Yield of 3: 1.18 g (...
example 2
The Compounds of Formula Ia
[0100]
may be prepared by the methods of known chemical reactions and procedures, some from starting materials which are well known in the art.
example 3
[0101]To screen the effectiveness and ideal doses for the HAOC (Heteroaromatic organic compounds) of the present invention at blocking SYN aggregation two sets of assays can be utilized. The first set involves in vitro assays in cell free and cell based systems and the second includes in vivo studies in transgenic mouse models of PD.
[0102]In the present example the following HAOC, denominated as NPT200-5, was used:
[0103]The objective is to identify with the in vitro assays a positive response by demonstrating a 50% effect in 2 out of the 3 assays at a 1 μM dose.
[0104]1. In Vitro Assays of SYN Aggregation and Toxicity
[0105]The in vitro studies include the following: i) effects on SYN oligomers in a cell free immunoblot assay of SYN aggregation; ii) effects on SYN accumulation and neurite outgrowth in neuronal cultures infected with a LV-SYN construct; and iii) effects on SYN oligomers in neuronal cultures infected with a LV-SYN construct.
[0106]For this purpose, recombinant SYN (1 μM,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com