Compositions and methods for reducing fucosylation of glycoproteins in insect cells and methods of use thereof for production of recombinant glycoproteins
a technology of recombinant glycoproteins and insect cells, which is applied in the field of molecular biology and the production of glycoproteins lacking fucosylation, can solve the problems of no reported efforts to block the fucosylation of recombinant glycoproteins in any insect-based system, and pre-existing human antibodies can give false positives, etc., to achieve the effect of stabilizing the inhibition of fucosylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
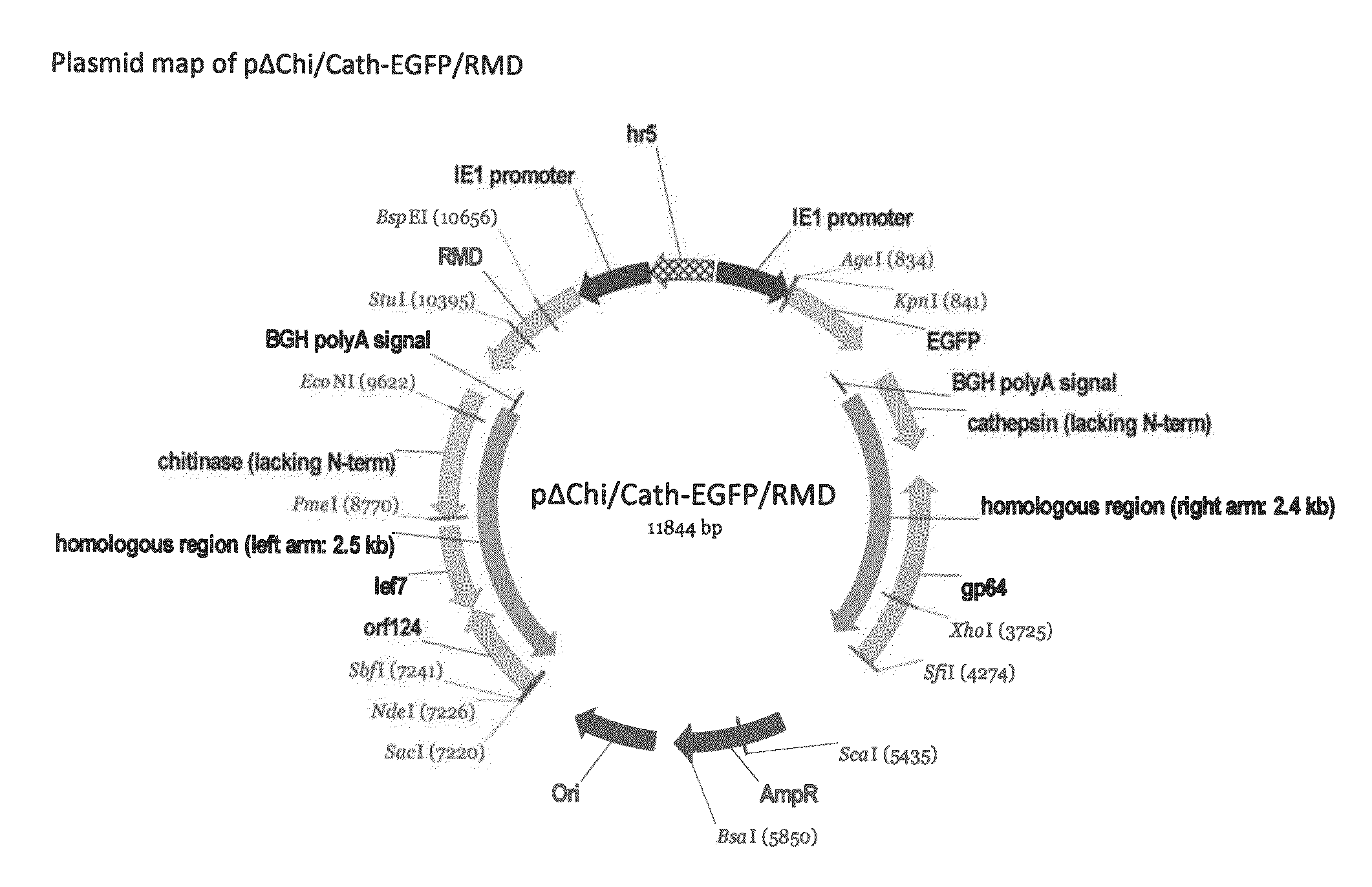
A Novel Baculovirus Vector for the Production of Non-Fucosylated Recombinant Glycoproteins in Insect Cells
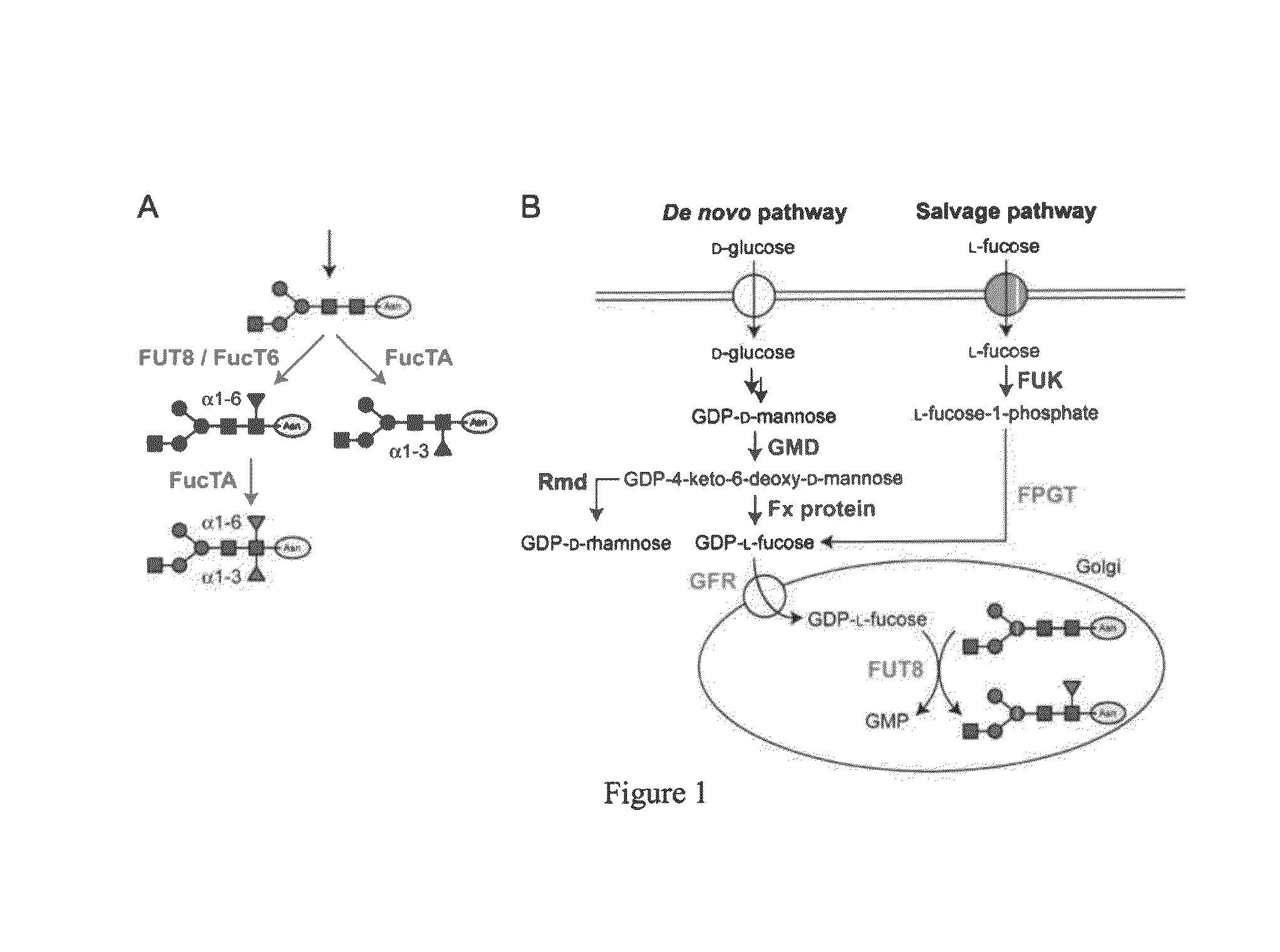
[0066]Analysis of Core α1,3-Fucosylation in Three Insect Cell Lines
[0067]High Five™ cells, derived from Trichoplusia ni, but not Sf9 cells, derived from Spodoptera frugiperda, produce core α1,3-fucosylated glycoproteins (Rudd et al. 2000; Seismann et al. 2010; Blank et al. 2011; Palmberger et al. 2011). Another Trichoplusia ni cell line used as a host for baculovirus expression vectors is Tni PRO™ (Kwon et al. 2009; Bourhis et al. 2010; Bongiovanni et al. 2012; He et al. 2013; Merchant et al. 2013), but its capacity for core α1,3-fucosylation has not been reported. Thus, we analyzed intracellular extracts of uninfected Tni PRO™ cells by western blotting with anti-horseradish peroxidase (HRP), which detects core α1,3-linked fucosylation, using extracts from Sf9 and High Five™ cells as negative and positive controls. Coomassie brilliant blue staining showed that approximately equa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com