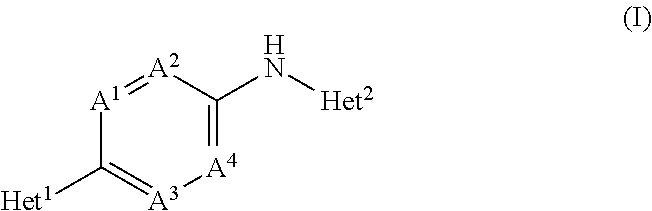
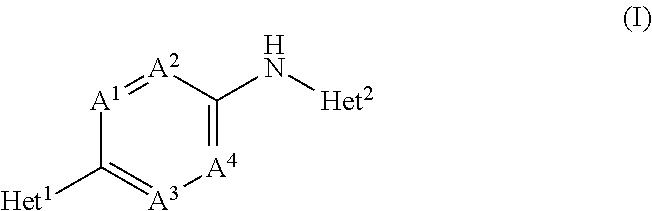
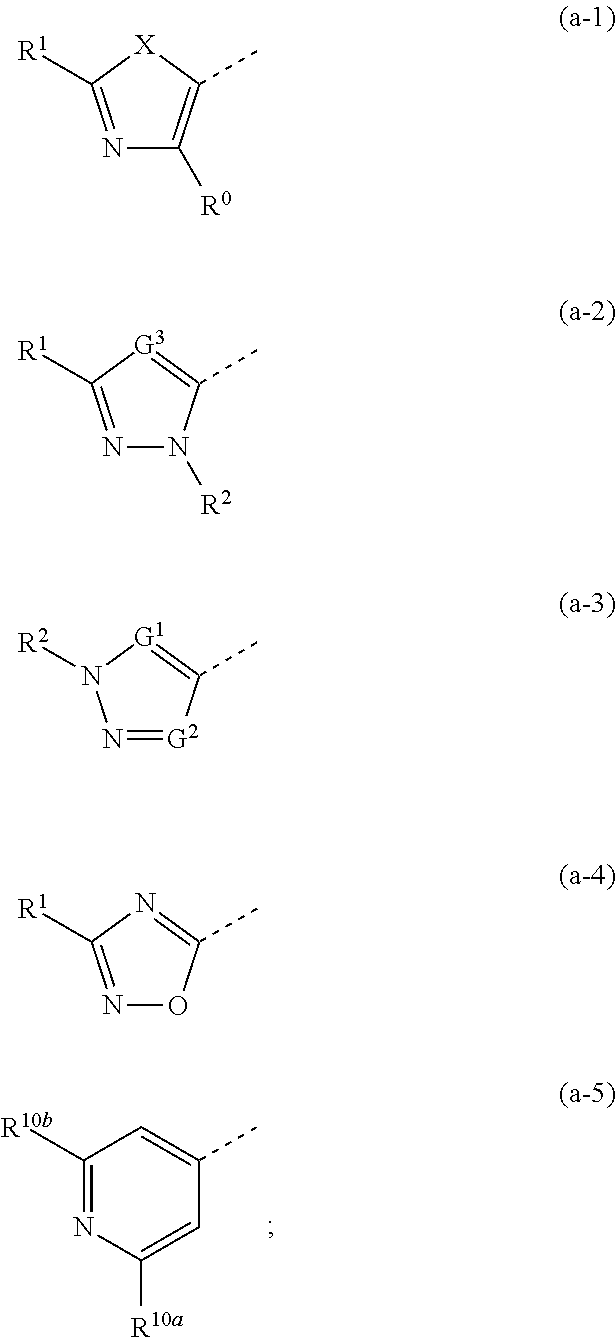
Novel substituted bicyclic heterocyclic compounds as gamma secretase modulators
a technology of gamma secretase and heterocyclic compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of low cns penetration, unwanted side effects, and no effective treatment of ad
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0435]Hereinafter, the term “DCM” means dichloromethane; “MeOH” means methanol; “LCMS” means Liquid Chromatography / Mass spectrometry; “aq.” means aqueous; “sat.” means saturated; “sol.” means solution; “HPLC” means high-performance liquid chromatography; “r.t.” means room temperature; “AcOH” means acetic acid; “m.p.” means melting point; “Et2O” means diethyl ether; “BDS” means base deactivated silica; “RP” means reversed phase; “min” means minute(s); “h” means hour(s); “I.D.” means internal diameter; “Pd(OAc)2” means palladium(II) acetate; “LiHMDS” means lithium hexamethyldisilazane; “HBTU” means 1-[bis(dimethylamino)methylene]-1H-benzotriazol-1-ium 3-oxide hexafluorophosphate; “Xantphos” means (9,9-dimethyl-9H-xanthene-4,5-diyl)bis[diphenylphosphine]; “X-Phos” means dicyclohexyl[2′,4′,6′-tris(1-methylethyl)[1,1′-biphenyl]-2-yl]-phosphine; “NH4OAc” means ammonium acetate; “NMP” means 1-methyl-2-pyrrolidinone; “SFC” means Supercritical Fluid Chromatography; “iPrNH2” means isopropylam...
example a1
a) Preparation of Intermediate 1
[0436]
[0437]K2CO3 (9.6 g, 69.5 mmol) and 1-methyl-1-tosylmethylisocyanide (8 g, 38.2 mmol) were added to a sol. of 2-formyl-5-nitroanisole (6.29 g, 34.7 mmol) in MeOH (150 ml) and the r.m. was refluxed for 4 h. The r.m. was concentrated under reduced pressure, the residue was dissolved in DCM and the organic phase was washed with H2O, dried (MgSO4), filtered and the solvent was evaporated in vacuo. The residue was purified by flash chromatography over Silica gel (eluent: n-heptane / EtOAc from 100 / 0 to 50 / 50). The product fractions were collected and the solvent was evaporated. Yield: 6.24 g of intermediate 1 (77%).
b) Preparation of Intermediate 2
[0438]
[0439]MeOH (150 ml) was added to Pd / C 10% (1 g) under a N2 atmosphere. Subsequently, a 0.4% thiophene sol. in DIPE (1 ml) and intermediate 1 (6.24 g, 26.6 mmol) were added. The r.m. was stirred at 25° C. under a H2 atmosphere until 3 eq of H2 was absorbed. The catalyst was filtered off over diatomaceous e...
example a2
a) Preparation of Intermediate 3
[0440]
[0441]Iodobenzene diacetate (5.49 g, 18.44 mmol) and trifluoromethanesulfonic acid (6.08 ml, 69.17 mmol) were stirred in CH3CN (100 ml) at r.t. for 1 h under N2. 2′-Methoxy-4′-nitro-acetophenone (3.0 g, 15.37 mmol) was added at once at r.t. to the sol. and the r.m. was then refluxed for 2 h, then cooled to r.t. and carefully added to a stirred sat. aq. sol. of Na2CO3 (500 ml). The product was extracted with DCM and the organic phase was dried (MgSO4), filtered and the solvent was evaporated under reduced pressure. The resulting dark brown oil was purified by flash column chromatography over Silica gel (eluent: DCM / MeOH isocratic 95 / 5). The product fractions were collected and the solvent was evaporated under reduced pressure. Yield: 3.0 g of intermediate 3 (75%).
b) Preparation of Intermediate 4
[0442]
[0443]MeOH (50 ml) was added to Pd / C 10% (0.250 g) under a N2 atmosphere. Subsequently, a 0.4% thiophene sol. in DIPE (2 ml) and intermediate 3 (0.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com