Systems and methods for multivariate analysis of adverse event data
a multi-variate analysis and data technology, applied in the field of systems, can solve the problems of inability to examine other factors, inability to analyze other factors, and inability to analyze other factors, and achieve the effects of avoiding the use of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
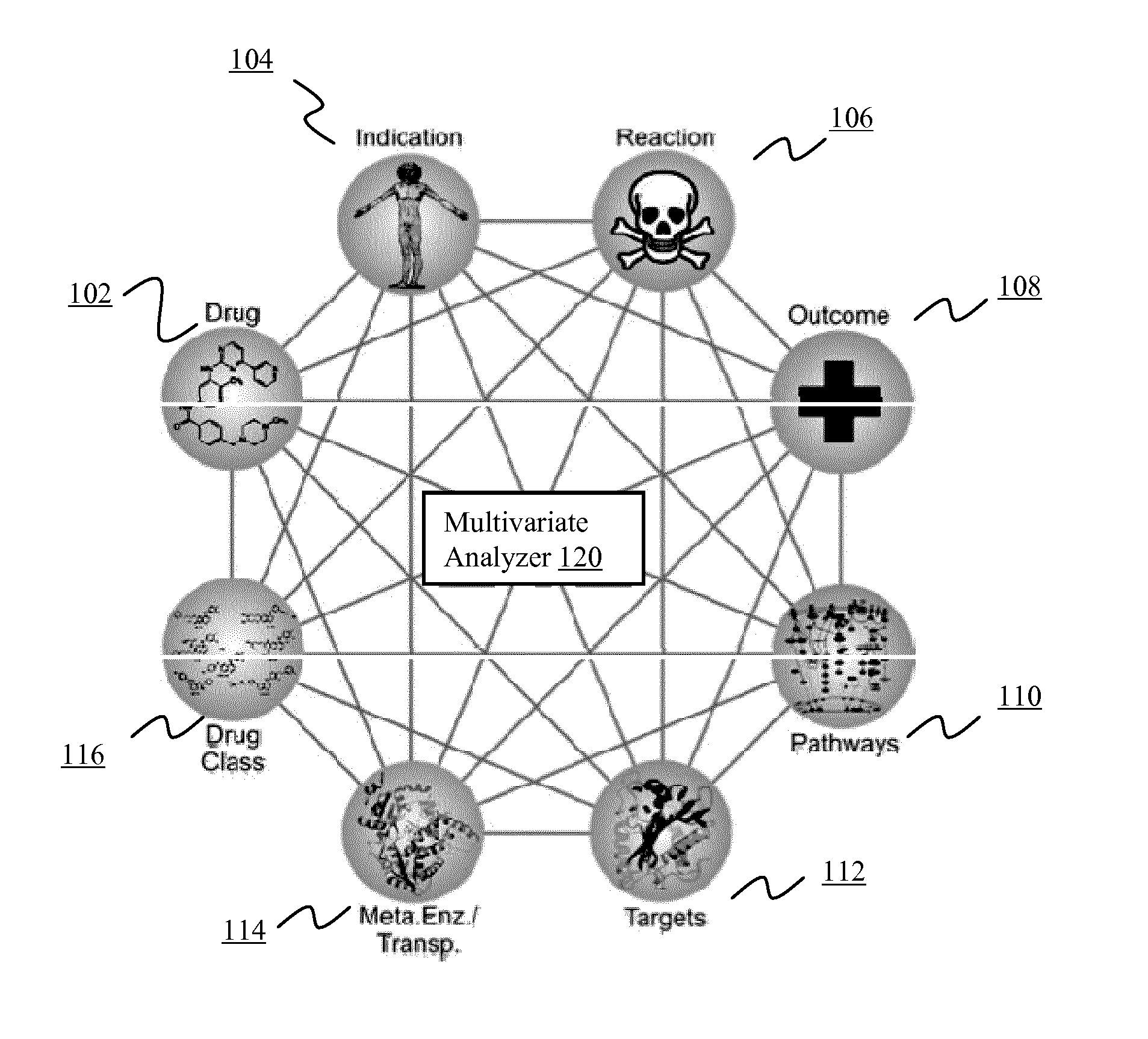
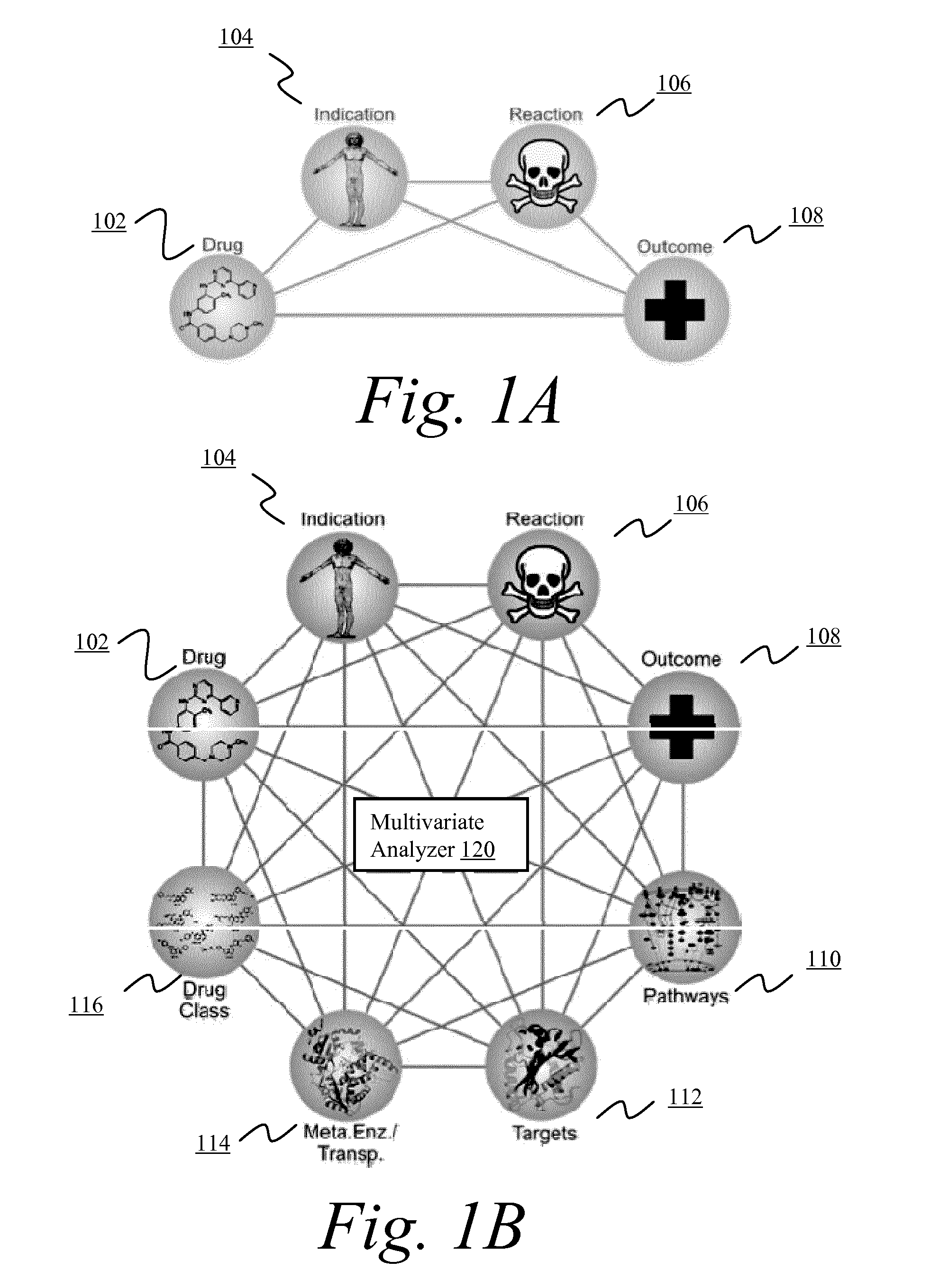
[0134]Adverse events are a common and, for the most part, unavoidable consequence of therapeutic intervention. The identification of novel adverse events is critical to the protection of patient well-being and the healthcare system that supports them. From the induction of avoidable and sometimes fatal side effects to the billions of dollars in associated medical costs, adverse events (AE's) remain a critical issue for all stakeholders in the healthcare system.
[0135]Data about adverse events are provided by clinicians, researchers, and manufacturers to spontaneous reporting systems, such as the U.S. Food and Drug Administration's Adverse Event Reporting System (AERS). After a manual review of each submission the data are made publically available on quarterly basis via the online AERS data files. All reports contain information surrounding the treatment, side effects, and patient characteristics / demographics. Drug information is further qualified as to whether the drug is suspected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com