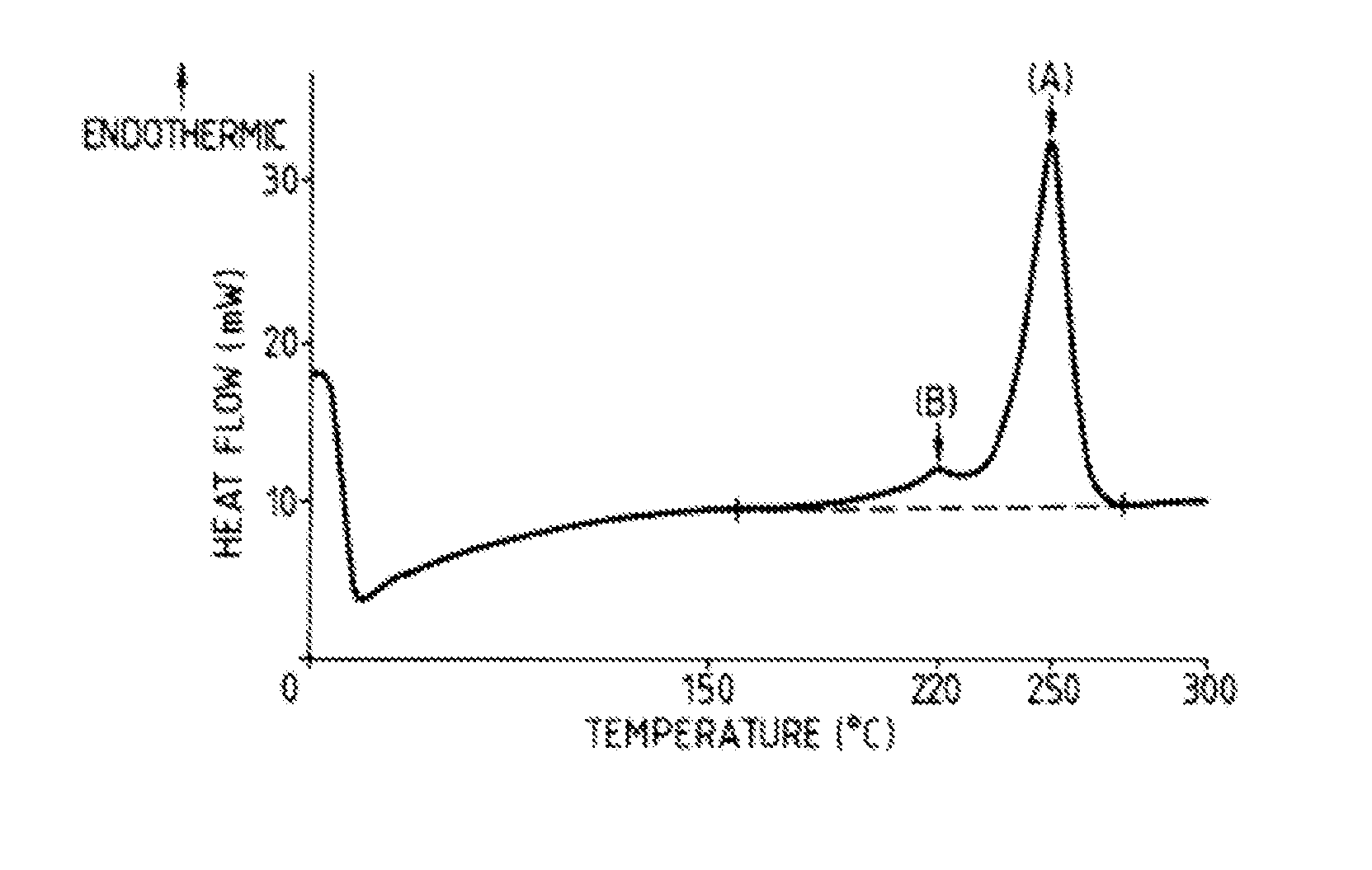
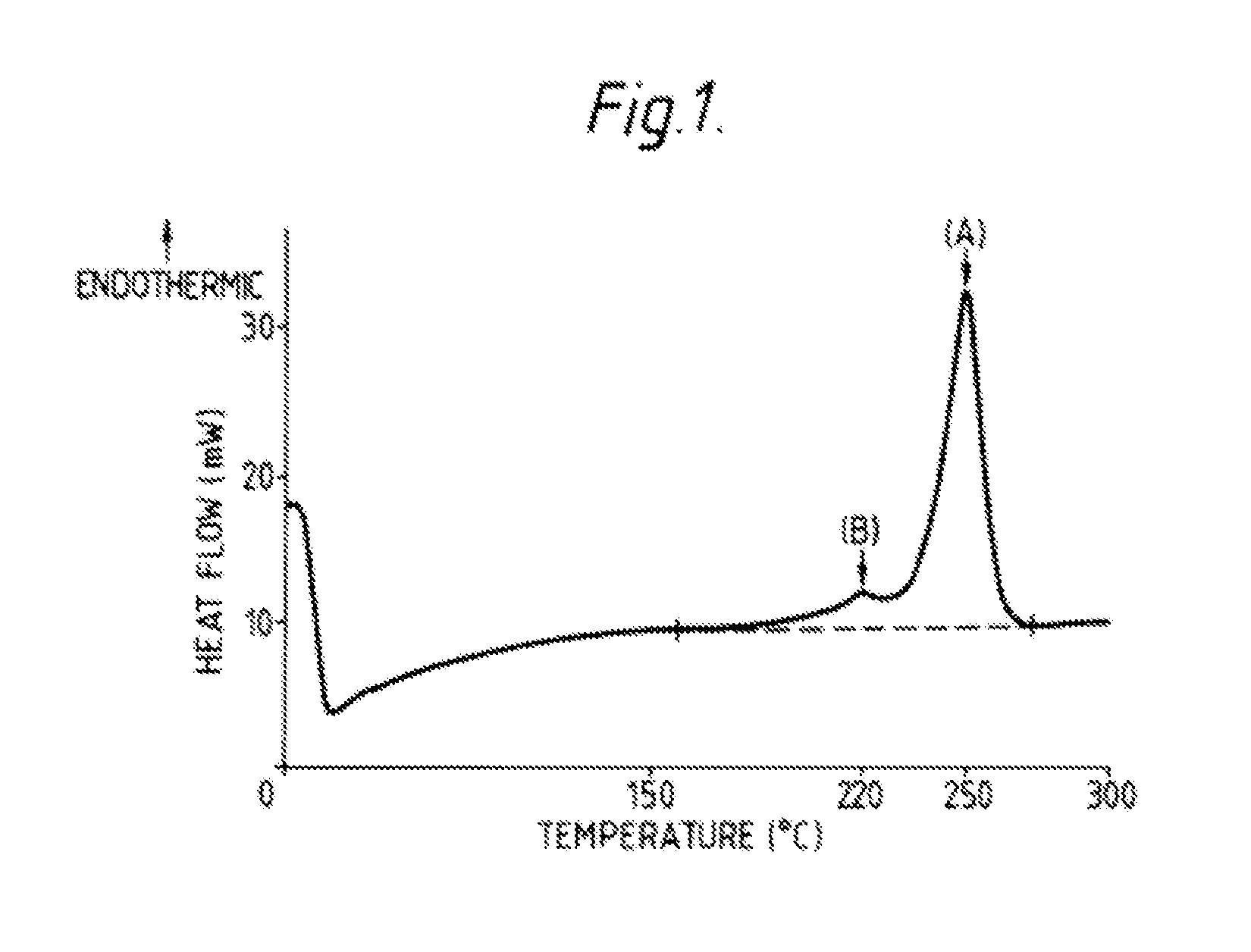
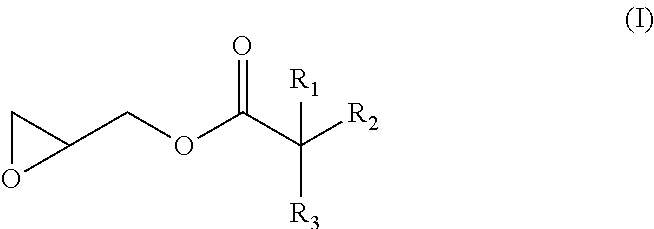
Hydrolysis resistant polyester films
a polyester film, hydrolysis resistant technology, applied in the field of polymer films, can solve the problems of reducing the intrinsic viscosity of the polymer, poor hydrolysis resistance, deterioration of one or more, etc., to improve the hydrolysis stability, improve product performance, and dissipate hea
Inactive Publication Date: 2015-06-04
DUPONT TEIJIN FILMS U S LLP
View PDF16 Cites 10 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
The present invention relates to a polyester film that has improved hydrolytic stability and can use reclaim film. The film is manufactured using a process route that minimizes carboxyl end-groups caused by thermal degradation during film manufacture. The use of a UV-absorber in the film provides improved UV-stability. The film can also incorporate an anti-oxidant to prevent degradation. The use of reclaim film and the addition of UV-absorber and anti-oxidant provide cost savings and improved performance.
Problems solved by technology
However, polyester films are susceptible to hydrolytic degradation, which results in a reduction in the intrinsic viscosity of the polymer, and a consequent deterioration in one or more of the afore-mentioned desirable properties of the film, particularly the mechanical properties.
Poor hydrolysis resistance is a particular problem when the film is used under humid conditions and / or elevated temperatures and / or in exterior applications, such as in photovoltaic (PV) cells.
For instance, the addition of carbodiimides as end-capping agents in polyester compositions was proposed in US-5885709 and EP-0838500, amongst others, but such additives have a tendency to emit harmful gaseous by-products.
One of the problems associated with the incorporation of hydrolysis stabilisers into polyester films is that while increasing the concentration of the additive improves the hydrolysis resistance, it does so at the expense of a reduction in the melting point and a deterioration in the mechanical properties of the polyester film.
One of the consequences of a reduction in mechanical properties is that the processability of the filmed polyester becomes poor, and breakage of the film web occurs during manufacture and subsequent processing.
Another problem with the use in the prior art polyester films of hydrolysis stabilisers based on epoxidised fatty acids, particularly epoxidised fatty acid glycerides, is that such additives have a tendency to decompose during film manufacturing and processing with evolution of acrolein, a highly toxic, flammable and foul-smelling substance.
An additional problem with the known hydrolysis stabilisers, particularly those based on certain epoxidised fatty acid glycerides and multi-functional glycidyl compounds, is the reduction of film quality and processability when such additives are incorporated into the film in an amount effective to provide improved hydrolysis resistance.
In particular, such additives induce profile defects and unacceptable levels of die-lines in polyester films, i.e. poor uniformity in thickness and / or light transmission across the film web, and the extrudate can become impossible to process on a film-line because of breakage of the film web.
It is believed that such problems are at least partly attributable to cross-linking and gel formation, which interferes with the stretching process experienced by the film during its manufacture.
A further problem with using multi-functional glycidyl compounds as hydrolysis stabilisers for PET is that their higher rate of chain extension of the polyester increases melt viscosity, which in turn reduces the extrusion output at a given temperature, and this is economically undesirable.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
examples
Control 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| heat-set | aaaaa | aaaaa |
Login to View More
Abstract
A biaxially oriented polyester film comprising polyester and at least one hydrolysis stabiliser selected from a glycidyl ester of a branched monocarboxylic acid, wherein the monocarboxylic acid has from 5 to 50 carbon atoms, wherein said hydrolysis stabiliser is present in the film in the form of its reaction product with at least some of the end-groups of said polyester, and wherein said reaction product is obtained by the reaction of the hydrolysis stabiliser with the end-groups of the polyester in the presence of a metal cation selected from the group consisting of Group I and Group II metal cations.
Description
[0001]This application is a continuation of U.S. application Ser. No. 14 / 003,676, filed 15 Nov. 2013, which is a National Phase filing of International Application No. PCT / GB2012 / 000224, filed 7 Mar. 2012, and which claims priority of GB Application No. 1103855.1, filed 7 Mar. 2011, and U.S. Provisional Application No. 61 / 449,897, filed 7 Mar. 2011, the entireties of which applications are incorporated herein by reference for all purposes.FIELD OF THE INVENTION[0002]The present invention is concerned with polyester films, particularly polyethylene terephthalate (PET) films, which exhibit improved hydrolysis resistance, and with a process for the production thereof.BACKGROUND OF THE INVENTION[0003]The advantageous mechanical properties, dimensional stability and optical properties of polyester films are well-known. However, polyester films are susceptible to hydrolytic degradation, which results in a reduction in the intrinsic viscosity of the polymer, and a consequent deterioration ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(United States)
IPC IPC(8): B29C47/00C08G63/91B29D7/01B29C48/08B29C48/18
CPCB29C47/0021B29K2067/003C08G63/916B29D7/01C08G63/914C08J5/18C08J2367/02Y02E10/52H01L31/049H01L31/056C08K3/22C08L33/02C08K3/013C08K3/014Y10T428/31786C08L67/02C08K5/1515B29C48/08B29C48/18B29C48/0018C08G63/91C08L67/00C08K5/10H01L31/048B29K2067/00C08J3/201C08J2367/03B32B2307/402B32B2367/00B32B2307/712B32B37/153B32B2307/412B32B27/08B32B2307/41B32B2250/03B32B27/18B32B2457/12B32B27/36B32B37/15B32B2250/244B32B2250/02B32B2307/50H01L21/64
Inventor BRENNAN, WILLIAM J.MORTLOCK, SIMON VERNONGOLDIE, WILLIAM BRYDENASHFORD, EMMAFORSYTH, KRISTIN JAYNETURNER, DAVID R.LOVATT, ALLAN
Owner DUPONT TEIJIN FILMS U S LLP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com