Methods for preventing and treating chronic kidney disease (CKD)
a kidney disease and chronic kidney disease technology, applied in immunological disorders, metabolism disorders, antibody medical ingredients, etc., can solve the problems of periostin, fibrosis and ckd that cannot be addressed, and achieve the effect of preventing or reducing renal fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
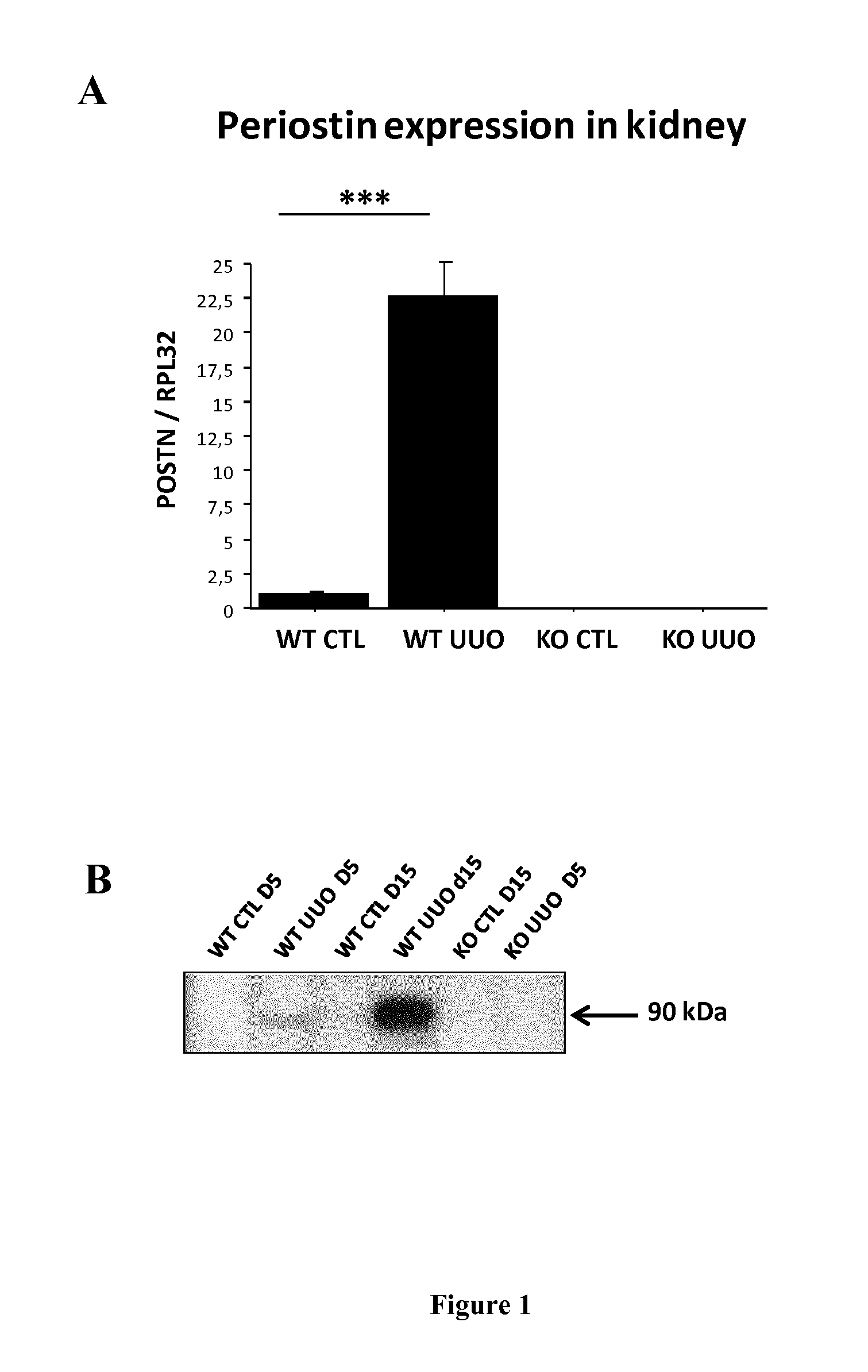
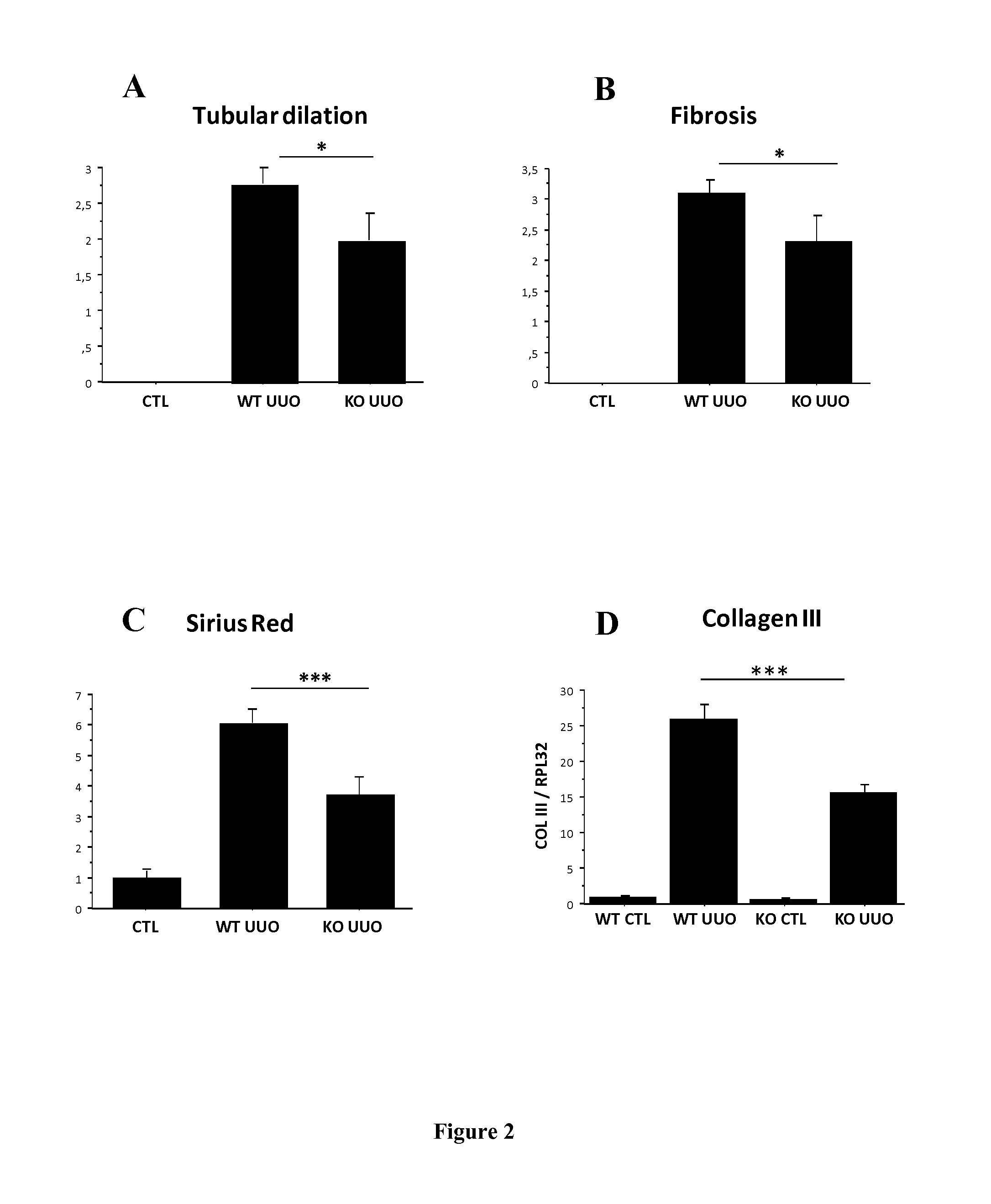
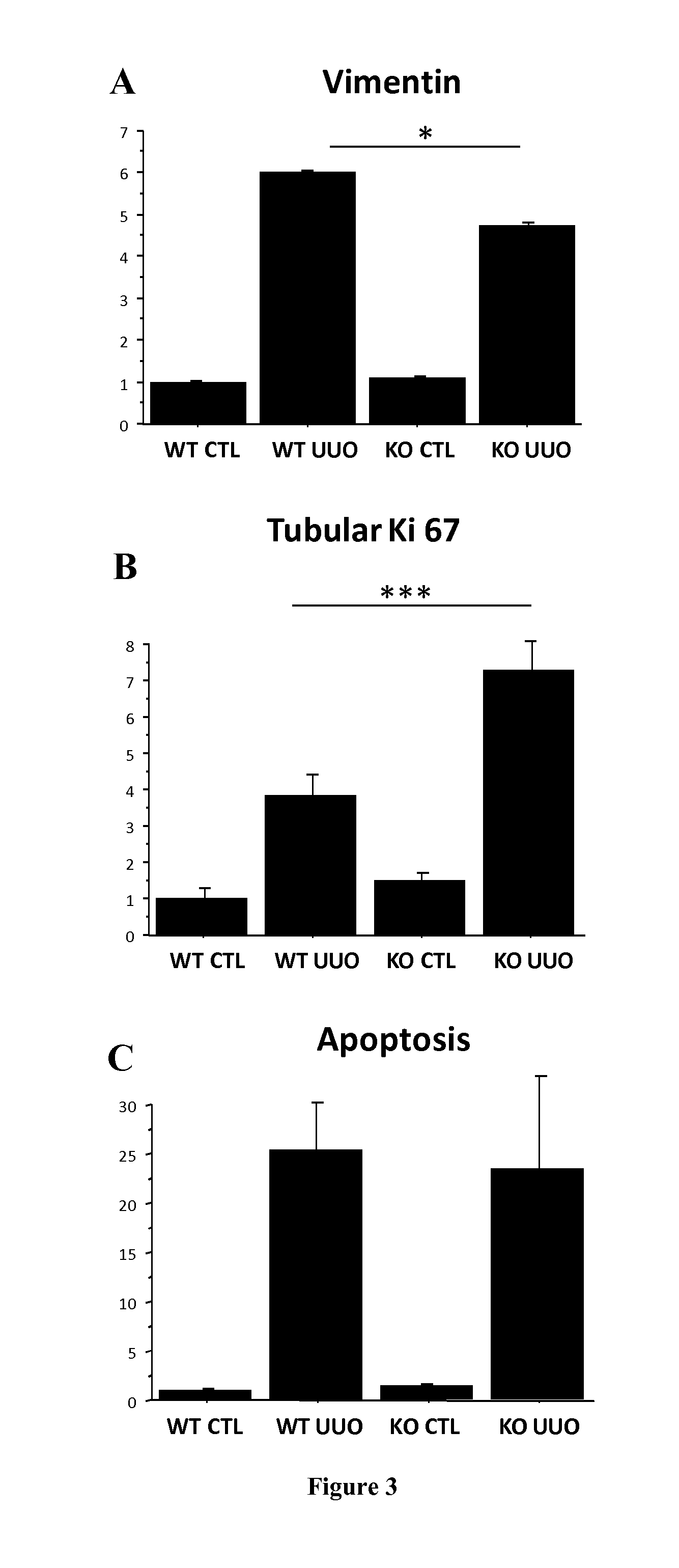
Periostin KO Mice are Protected Against the Development of CKD in an Experimental Tubulointerstitial Nephropathy (UUO Model)
[0090]Material & Methods
[0091]Animals:
[0092]Experiments were performed on wild type and periostin null mice (POSTN KO) donated kindly by S.J. Conway (Indianapolis, Ind.). These mice are characterized by the lack of the periostin gene and the replacement of the translation start site and the first exon by a lacZ reporter gene (ref). C57BL / 6 WT and POSTN KO female mice aged 4 to 6 months were used (n=9). Tubulointerstitial nephropathy model was induced by the unilateral ureteral obstruction (UUO). After induction of general anesthesia (intraperitoneal injection of pentobarbital 50 mg / kg), POSTN KO mice and WT counterparts were subjected to a left flank incision. UUO was performed by complete ligation of the left ureter at the ureteropelvic junction, using double silk sutures. Controlateral kidneys were used as controls. Mice were sacrificed at day 15.
[0093]Animal...
example 2
Periostin Antisens Oligonucleotide Periostin Protect Against the Development of in a Hypertensive Nephropathy Rat Model (L-NAME Treated Rats)
[0119]Material & Methods
[0120]Animals:
[0121]Male Sprague-Dawley rats, weighing 250 g, were maintained on a normal-salt diet and had free access to chow and water. To induce a hypertensive nephropathy model, LNAME, an inhibitor of NO synthesis, was given via drinking water at the dose of 30 mg / kg / day. In order to investigate the role of periostin, the inventors used an antisense approach to down regulate its expression.
[0122]Administration of Antisense (AS) Against Periostin:
[0123]To block periostin expression, the inventors used a specific AS oligodeoxynucleotides (ODN) modified with phosphorothioate to prevent their in vivo hydrolysis by nucleases. The AS or scrambled control ODNs were administrated by intraperitoneal (IP, 1 μM) either in a preventive or curative way. In the preventive protocol, antisense injection started with LNAME administr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene expression profile | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com