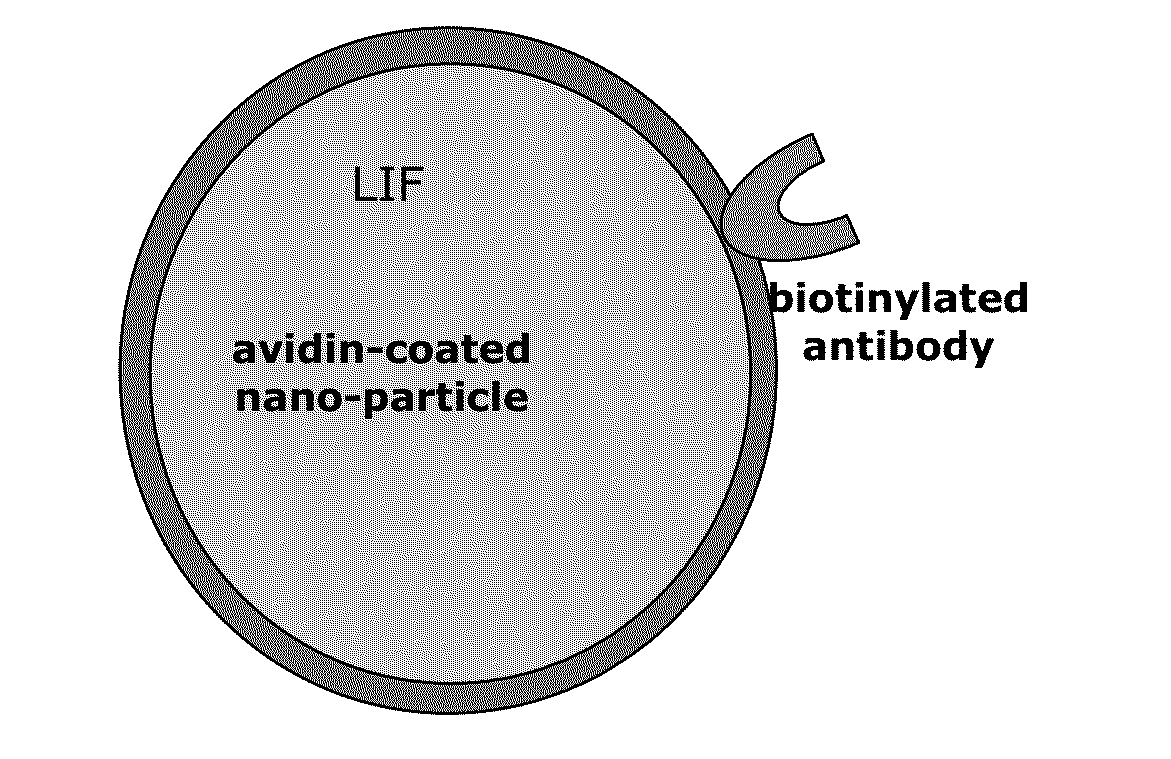
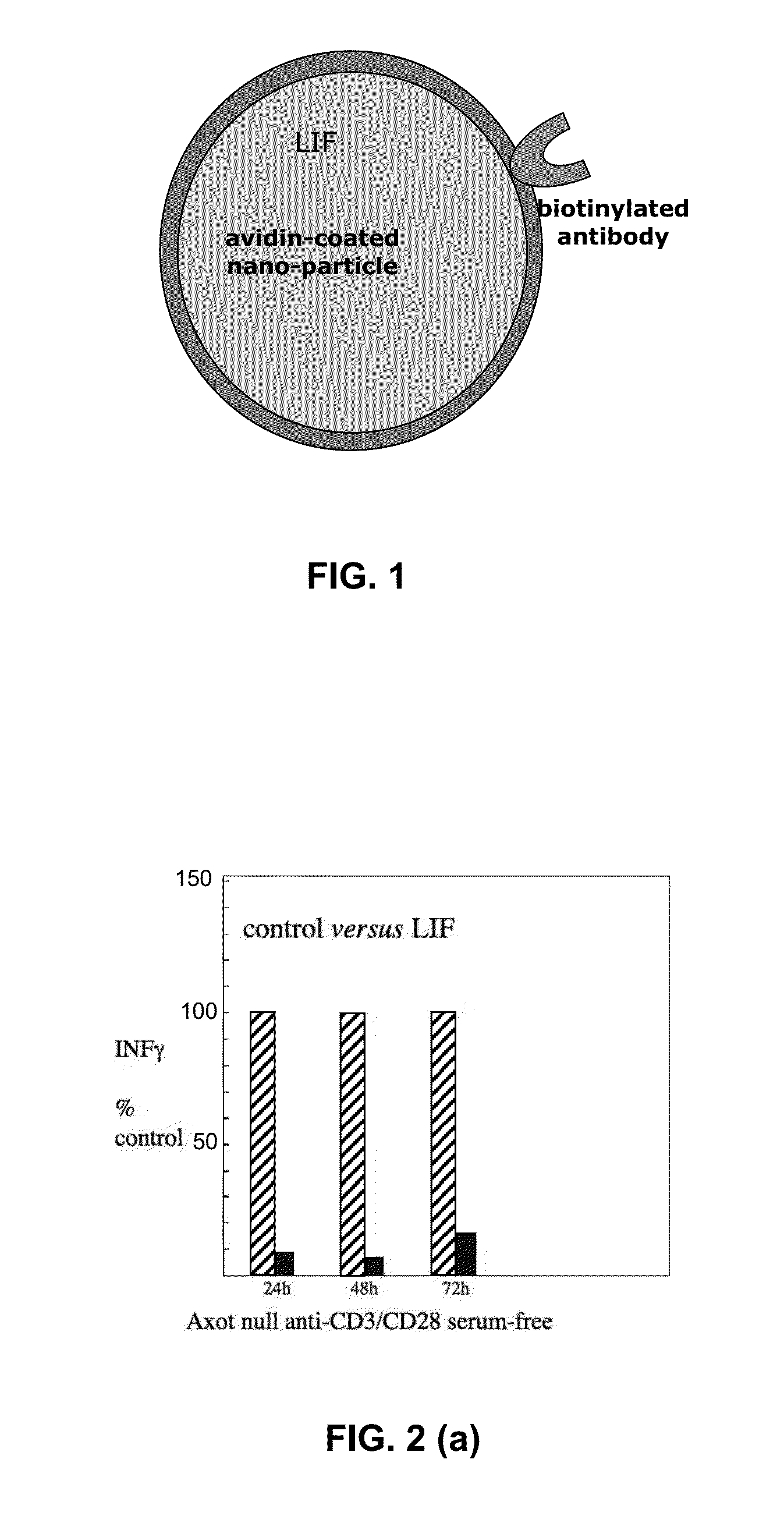
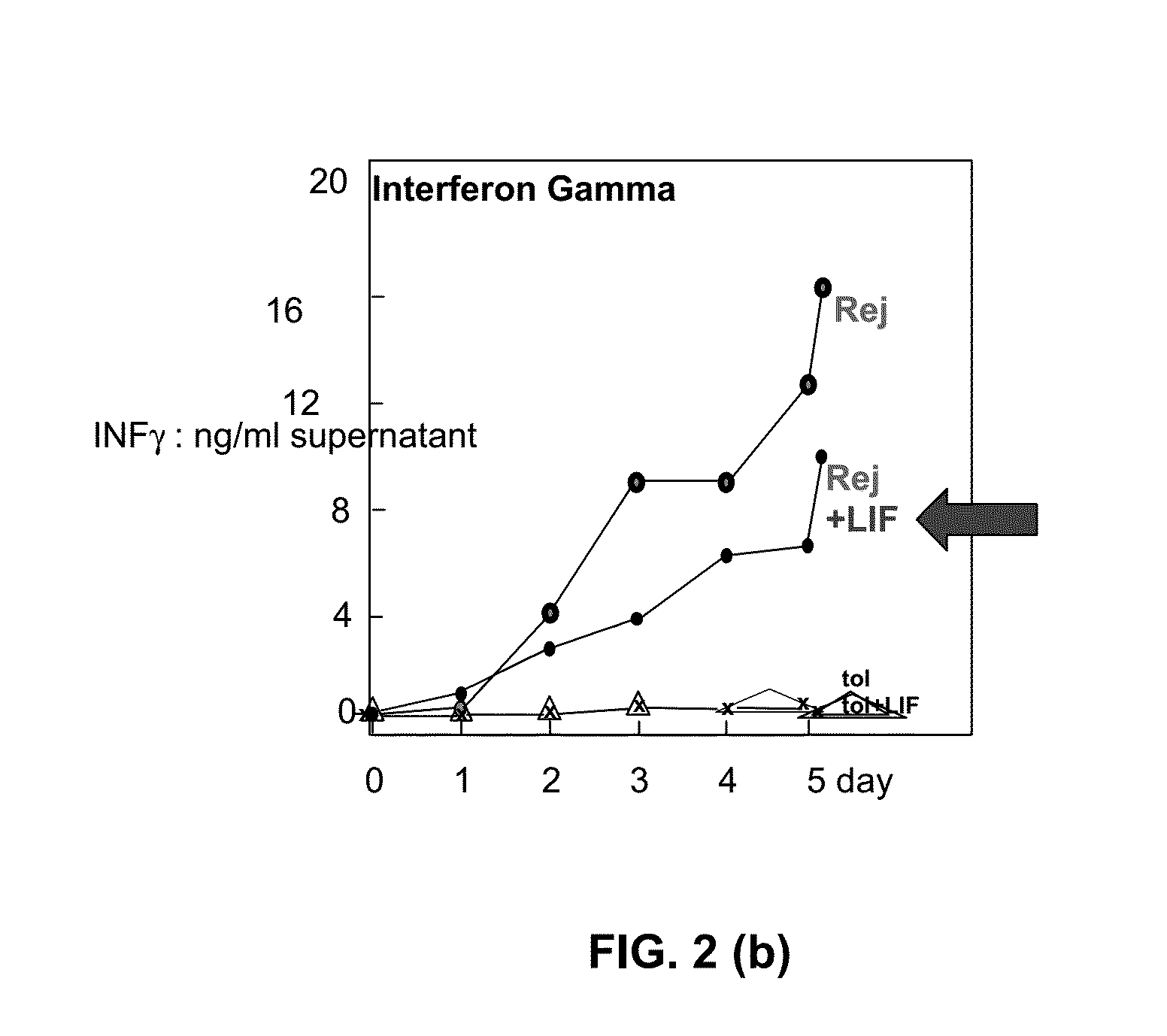
Immuno-modulatory composition
a technology of immunomodulatory composition and composition, applied in the field of immunomodulatory composition, can solve the problems of poorer candidates, patient death, organ failure, etc., and achieve the effect of reducing aggressive immune activity and considerable clinical benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0037]Experimental models in mice have identified a critical regulatory system in T lymphocytes wherein Foxp3—the master gene for regulatory tolerance—is itself regulated by axotrophin / MARCH-7 (an E3-ligase) and LIF. Not only does axotrophin / MARCH-7 directly regulate LIF release by T lymphocytes, but also both axotrophin / MARCH-7 and LIF are required for normal Foxp3 gene activity.
[0038]In humans, Foxp3 and axotrophin / MARCH-7 are co-expressed in peripheral blood cells. In patients who have received a bone marrow transplant, and also in patients who have received a kidney transplant, expression of Foxp3 and axotrophin / MARCH-7 positively correlate with good graft function (Muthukumarana et al 2007). These findings infer that the relationship between immune tolerance, axotrophin / MARCH-7 and—by extrapolation—LIF is valid in clinical patients and supports the present invention which adopts a novel tolerogenic therapeutic approach of targeted delivery of LIF to the site of immune activity....
example 2
[0089]This Example shows the effect of exogenous LIF on T cells activated by CD3 / CD8 ligation.
[0090]Genetically normal mouse spleen cells cultured in serum-free growth medium were activated by anti-CD3 and anti-CD28 in the presence or absence of 10 ng / ml LIF. As shown in FIG. 3, at 48 h exogenous LIF was found to have increased expression of Nanog, p53, and LIF genes, and decreased SOCS-3 gene expression.
[0091]It is therefore predicted that in vivo therapy using targeted delivery of LIF will similarly increase the expression of Nanog, p53, and LIF genes in a target cell, and depress SOCS-3 expression.
example 3
[0092]This Example shows that cell-derived LIF protein is linked to allo-tolerance, whereas release of IL6 protein is linked to allo-rejection.
[0093]Spleen cells from in vivo primed allo-tolerant, or allo-rejected, mice stimulated by donor antigen (i.e. irradiated donor-type spleen cells) in RPMI growth medium containing 10% FCS revealed that release of cell-derived LIF protein is linked to allo-tolerance, whereas release of IL6 protein is linked to allo-rejection (see FIG. 4).
[0094]It is therefore predicted that targeted delivery of LIF to CD4+ T cells in vivo will result in increases in the regulatory tolerant (Treg) lineage, whereas targeted delivery of IL6 will oppose induction of tolerance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| cell surface | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com