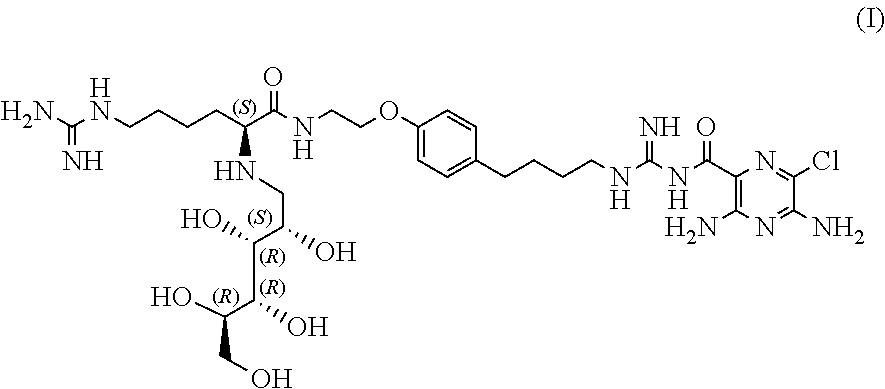
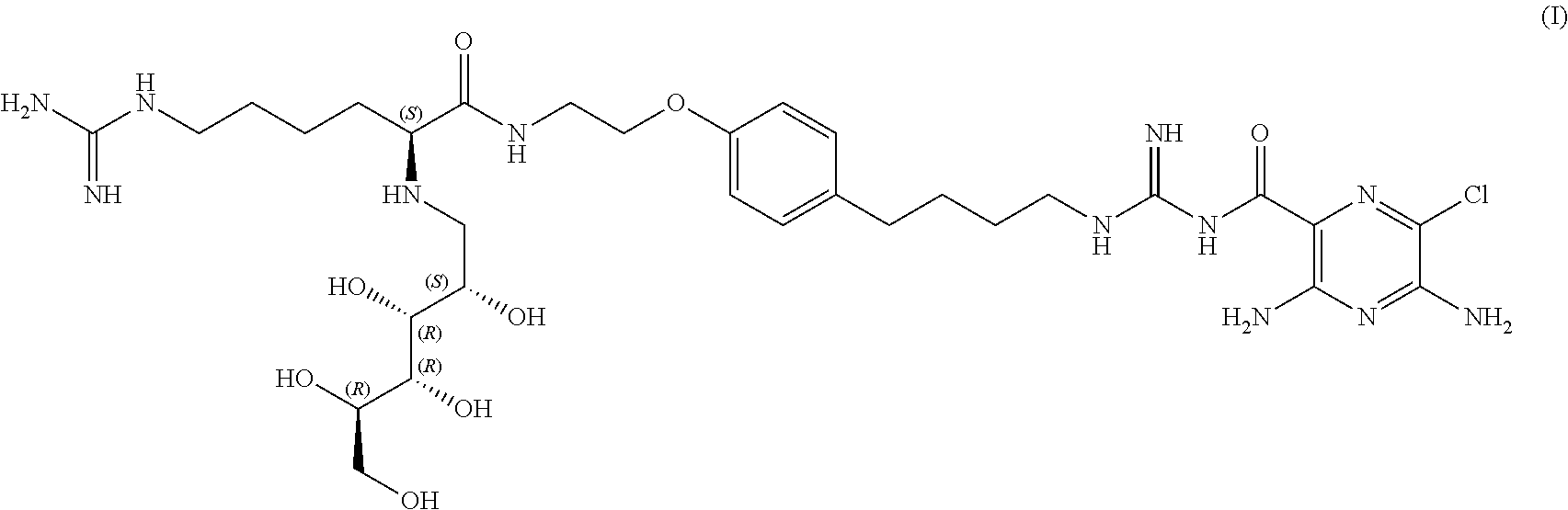
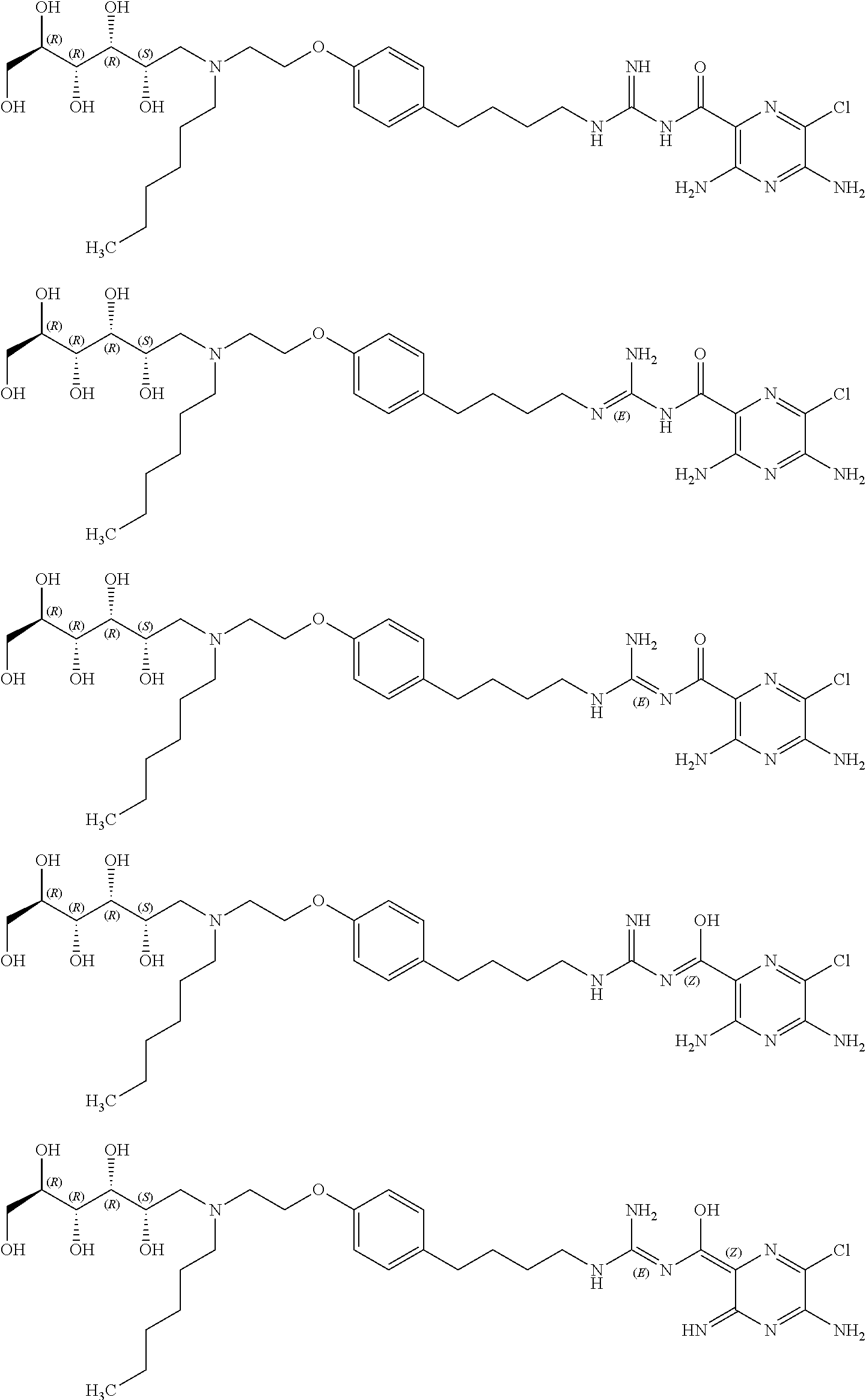
Stable sodium channel blockers
a sodium channel blocker and stable technology, applied in the field of epithelial sodium channel blockers, can solve the problems of hyperkalemia and death, and achieve the effect of slow reversibility and slow absorbing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compound 13
[0165]To a solution of benzyl 4-(4-hydroxyphenyl)butylcarbamate (11, 10.0 g, 33.4 mmol) in dry THF (100 mL) was added N-Boc ethanolamine (12, 13.4 g, 83.5 mmol), Ph3P (21.8 g, 83.5 mmol) and DIAD (16.8 g, 83.5 mmol) at 0° C., then the reaction mixture was warmed to room temperature and stirred over night. The reaction mixture was concentrated in vacuum and the residue was purified by column (silica gel, 95:5 CHCl3 / MeOH) to afford compound 3 (9.0 g, 64%) as a yellow solid: 1H NMR (400 MHz, CDCl3) δ 7.35-7.34 (m, 5H), 7.10 (d, J=8.0 Hz, 2H), 6.80 (d, J=8.0 Hz, 2H), 5.10 (s, J=4.0 Hz, 2H), 4.01-3.98 (m, 2H), 3.5 (q, 2H), 3.2 (q, 2H), 2.55 (t, J=8.0 Hz, 2H), 1.60-1.49 (m, 4H), 1.45 (s, 9H).
example 2
Preparation of Benzyl 4-(4-(2-aminoethoxyl)phenyl)butylcarbamate Hydrochloric Acid Salt (14)
[0166]Compound 13 (9.0 g, 20.3 mmol) was dissolved in 4 N HCl in dioxane (50 mL) at room temperature and the solution was stirred for 1 h. After concentrated, the residue was suspended in MTBE (250 mL) and stirred for 0.5 h. The solid is filtered out to afford hydrochloric acid salt 14 (7.6 g, 90%) as a white solid: 1H NMR (300 MHz, CD3OD) δ 7.33-7.32 (m, 5H), 7.10 (d, J=8.7 Hz, 2H), 6.88 (d, J=8.7 Hz, 2H), 5.05 (s, 2H), 4.18 (t, 2H), 3.39-3.31 (m, 2H), 3.11 (t, J=7.2 Hz, 2H), 2.56 (t, J=7.5 Hz, 2H), 1.59-1.49 (m, 4H).
example 3
Preparation of Compound 16
[0167]To a solution of compound 14 (5.6 g, 21.8 mmol) in dry THF (250 mL) was added DIPEA (12.0 mL, 65.4 mmol), DEPBT (8.4 g, 28.3 mmol) and compound 15 (11.1 g, 23.9 mmol). The reaction mixture was stirred at room temperature overnight and concentrated in vacuum. The residue was purified by column chromatography (silica gel, 95:5 CHCl3 / MeOH) to afford desired compound 16 (7.6 g, 71%) as a white solid: 1H NMR (400 MHz, CD3OD) δ 7.75 (d, J=7.4 Hz, 2H), 7.63 (d, J=7.4 Hz, 2H), 7.39-7.26 (m, 9H), 7.00 (d, J=8.0 Hz, 2H), 6.76 (d, J=8.0 Hz, 2H), 5.04 (s, 2H), 4.32 (d, J=6.4 Hz, 2H), 4.16 (t, J=6.8 Hz, 1H), 4.04 (t, J=8.0 Hz, 1H), 3.97 (t, J=5.2 Hz, 2H), 3.60-3.49 (m, 2H), 3.09 (t, J=6.8 Hz, 2H), 2.97 (t, J=6.4 Hz, 2H), 2.48-2.47 (m, 2H), 1.72-1.30 (m, 19H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Hydration number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com