HIV Antigens and Antibodies
a technology of antigens and antibodies, applied in the field of hiv antigens and antibodies, can solve the problems of inability to cure hiv-1 infection, inability to effectively prevent infection, and limited current treatment options for hiv-infected individuals, so as to reduce hiv-1 transmission and disease progression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Manufacture of the Virus Particle Product
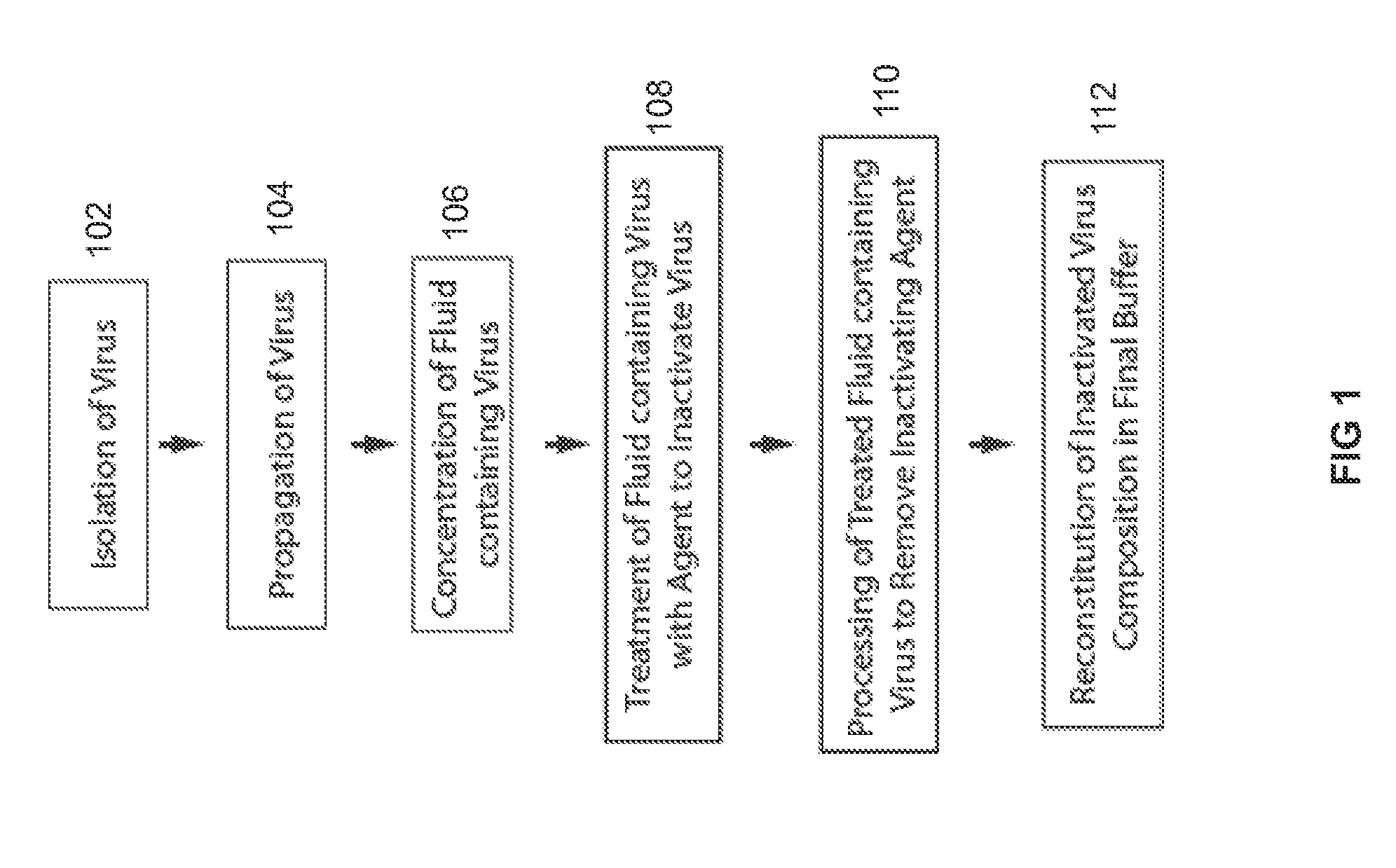
[0138]Those of ordinary skill in the art can appreciate that there are many processes employed in the production of the non-infectious virus particle product that retains trimeric envelope glycoprotein subunits (TEGS) in their natural state. While TEGS is the virus particle product in the following examples, other virus particle products may also be produced and used. In a preferred embodiment, infectious virus stocks were prepared from plasma in mitogen-stimulated PBMC or CD4+ blood cells (Vyas G N. Human peripheral blood mononuclear cell substrate for propagating wild type HIV-1, Dev Biol (Basel) 2001 106:345-356), purified and concentrated, and then inactivated by treatment to deplete membrane cholesterol, capsid proteins, and RNA (FIG. 1).
example 2
Production of High-Titer HIV-1 Stocks
[0139]The production of the viral particle product requires appreciable amounts of infectious virus. In a preferred embodiment, high-titer stocks of HIV were produced using HIV isolated from HIV-infected individuals who were in the earliest stage of infection prior to antibody detection. Such viruses have been called ‘Fiebig I / II’ isolates or ‘founder’ viruses (Fiebig E W, et al., AIDS 2003, 17:1871-1879; Keele B F et al. Proc Nat'l Acad Sci 2008, 105: 7552-7557). The expansion of virus was performed using primary blood cells that can be obtained from blood banks while preserving the anonymity of the blood donors. Primary blood cells were used to expand the virus in its most natural form, as opposed to the use of cell lines that produce variants of the original virus.
[0140]Peripheral blood mononuclear cells (PBMCs) were isolated by routine Ficoll density gradient separation procedures (Vyas G N et al., Biologicals, 2012 40: 15-20). To further pur...
example 3
Measuring Viruses and Virus Particle Products
[0144]The assay most widely used in research laboratories for measuring HIV levels is an ELISA that detects the p24 capsid protein. The p24 protein is the most abundantly produced virus protein in infected cells and is the most abundant protein in the virus. Thus, the p24 ELISA is a useful assay for detecting the presence of HIV. However, because the majority of p24 is not incorporated into the virus and because p24 is present in defective viruses, the p24 ELISA does not reliably measure infectious viruses. An alternative assay measures levels of the virus reverse transcriptase protein. The present application embodies a preferred method for measuring virus reverse transcriptase: a one-step quantitative non-radioactive RT-PCR method. The method involved pelleting virus from an aliquot of the sample to be tested, lysis of the pelleted virions, the addition of a reaction mixture, incubations of varying lengths of time at various temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com