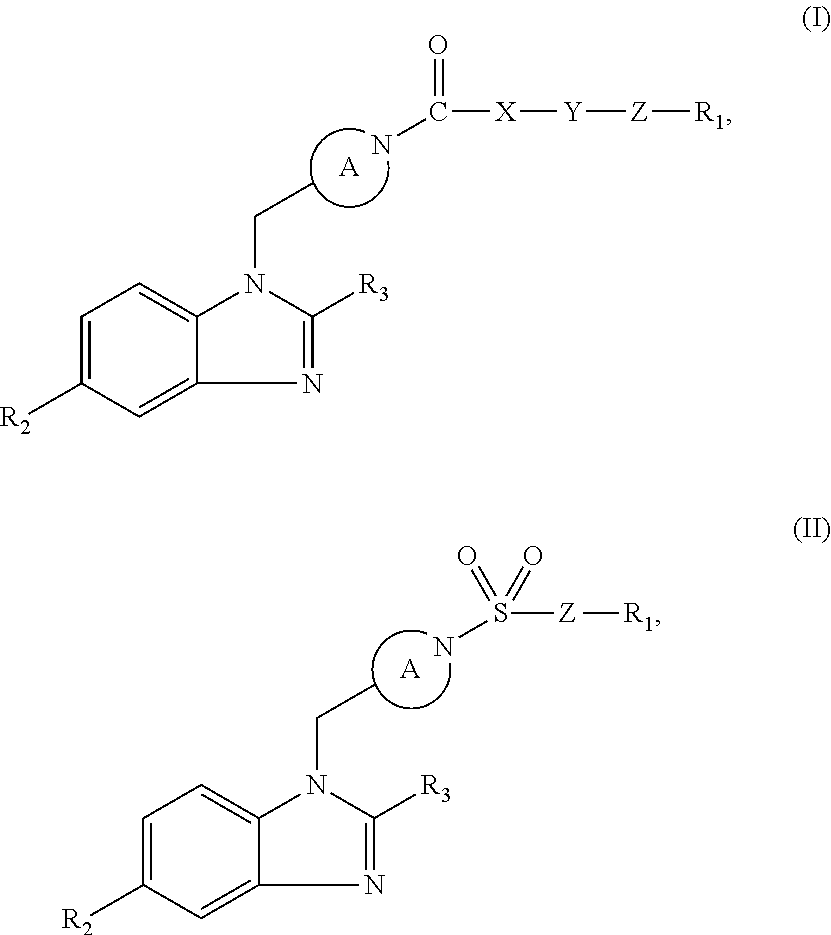
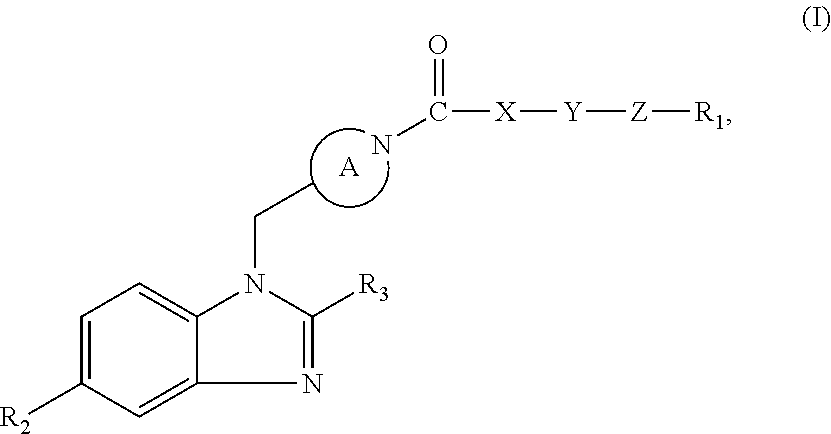
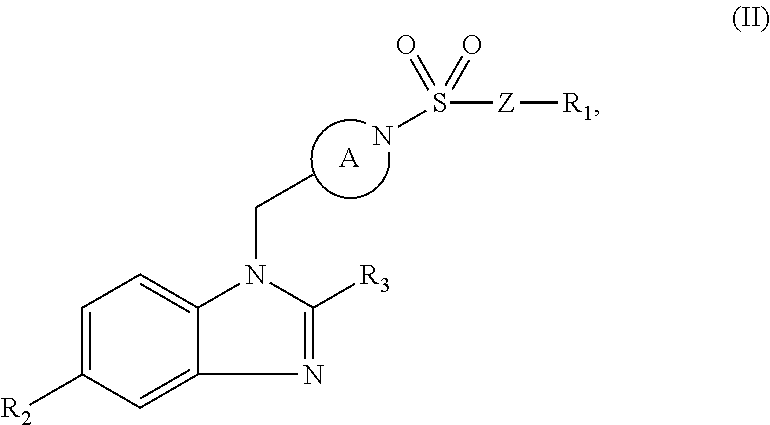
Benzimidazole retinoic acid receptor-related orphan receptor modulators and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of phenyl(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazol-1-yl)methyl)piperidin-1-yl)methanone
[0301]
[0302]To a stirred solution of 1-(piperidin-4-ylmethyl)-5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazole (0.200 g, 0.54 mmol, 1.0 eq) in dichloromethane (5 mL), benzoyl chloride (0.101 g, 0.65 mmol, 1.2 eq) was added at 0° C. followed by triethylamine (0.163 g, 1.62 mmol, 3.0 eq) and the mixture was stirred room temperature for 1 h. After completion of the reaction (monitored by TLC, 20% ethyl acetate in hexanes Rf=0.55), water (10 mL) was added to the reaction mixture and it was extracted with dichloromethane (10 mL). The organic extract was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain a crude product, which was purified by column chromatography on silica gel, 100-200 mesh, eluting with 10% ethyl acetate in hexanes to afford phenyl(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-...
examples 2-4
[0303]The above experimental protocol was used for synthesis of the compounds shown in the table below:
YieldExampleIntermediateg, (%)LCMS20.12 (80)Purity: 59.18% (ES+) m / z 484.50; tr = 1.56 min. 2-Phenyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazol-1-yl)methyl)piperidin-1-yl)ethanone30.06 (41)Purity: 88.55% (ES+) m / z 498.28; tr = 1.59 min. 3-Phenyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazol-1-yl)methyl)piperidin-1-yl)propan-1-one40.077 (52)Purity: 90.53% (ES+) m / z 490.33 (M + H+); tr = 1.71 min. 2-Cyclohexyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazol-1-yl)methyl)piperidin-1-yl)ethanone
example 5
Synthesis of 1-((1-(phenylsulfonyl)piperidin-4-yl)methyl)-5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazole
[0304]
[0305]1-((1-(Phenylsulfonyl)piperidin-4-yl)methyl)-5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazole was prepared from 1-(piperidin-4-ylmethyl)-5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-benzo[d]imidazole and benzene sulfonyl chloride using the procedure described in Example 1 to give a 27% yield of product. Purity: 60.80%; (ES+) m / z 506.16 (M+H−); tr=1.65 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com