Therapeutical composition containing apomorphine as active ingredient
a technology of active ingredients and therapeutic compositions, applied in the direction of pharmaceutical delivery mechanisms, medical preparations, nervous disorders, etc., can solve the problems of inducing injection site reactions, local subcutaneous site reactions, situ precipitation and chemical degradation under physiological conditions, etc., to increase the local tolerance of treatment, increase the chemical stability of formulations, and increase drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 4
Local Tolerance
[0122]The local tolerance results of the tested formulations in rats (1 mg / kg—SC) are presented in the Table 3.
TABLE 3ParameterAPO-go ®Test 1Test 2Test 3 Test 4Test 5Concentration (mg / ml)5.029.110.029.429.429.4pH3.35.94.26.05.94.1Toleranceformulation2.72.43.72.33.03.2scoringplacebo0.30.60.50.30.20.8Note:tolerance scoring for the saline solution was 0.1.
[0123]The same tolerance level as the Apo-go® reference is observed for the 5 test formulations, which have a concentration from 2 to 6 times higher than the reference (from 10 to 30 mg / mL for the test formulations vs. 5 mg / mL for the reference).
[0124]Thus, using a formulation according to the present invention, patients will be able to receive a better treatment based on a 6-fold lower injection volume, a formulation at a more physiologically acceptable pH and a formulation that tends to be more tolerated than the APO-go® reference.
example 5
14-Day Subcutaneous Infusion in Vivo Testing
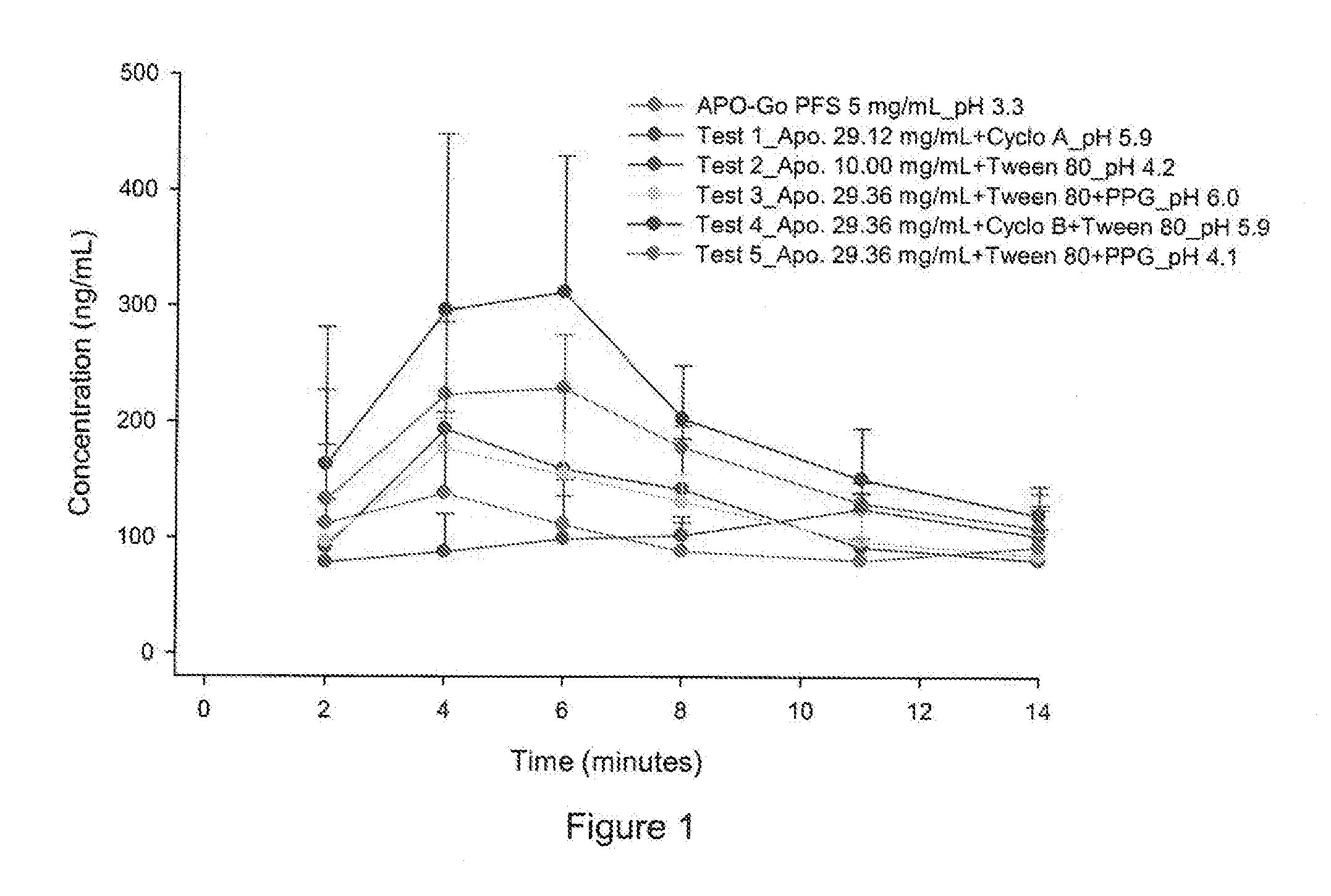
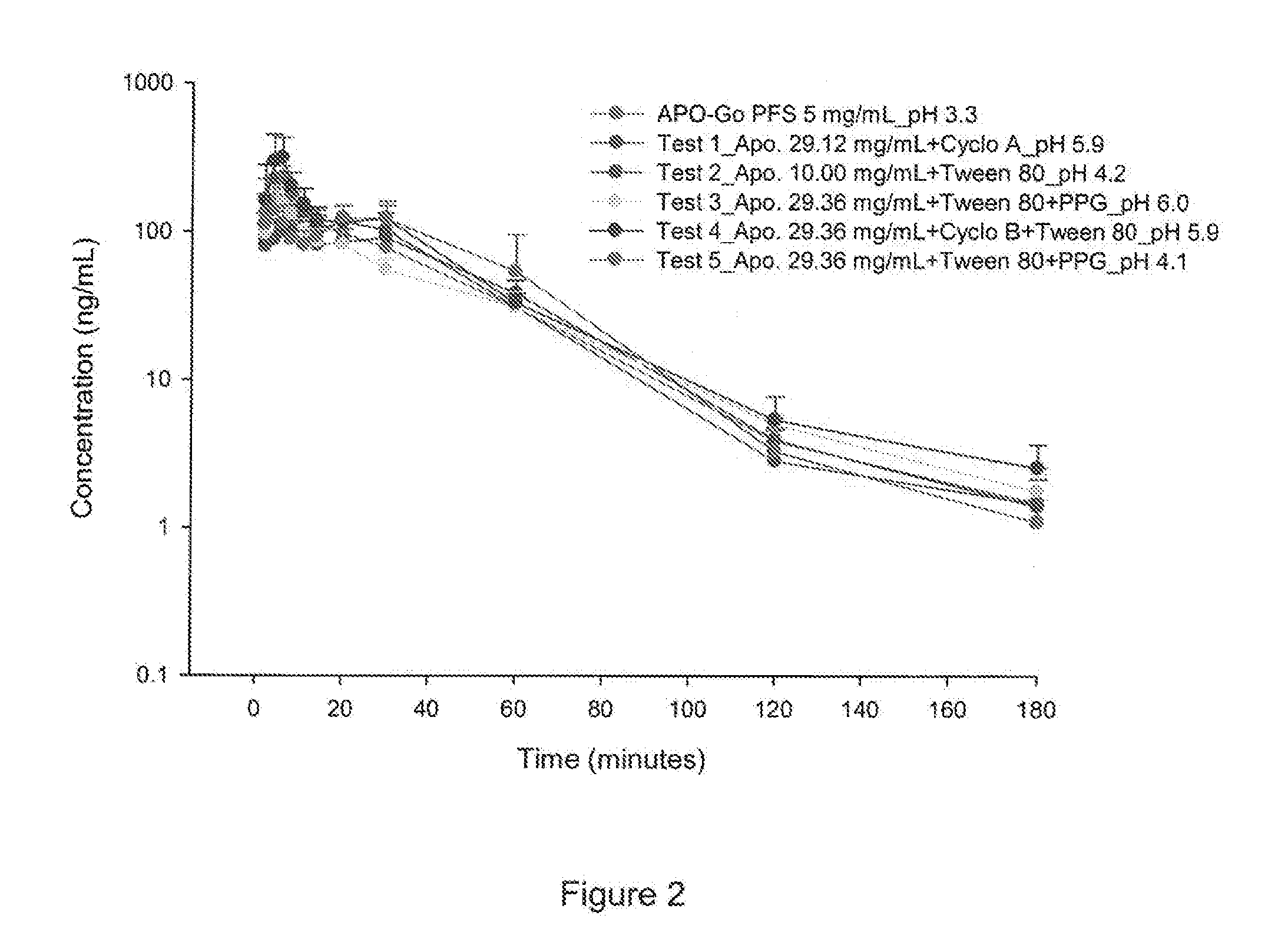
[0125]PK profile of the selected prototypes were evaluated in rats. Subcutaneous micropumps were implanted in 6 rats for each prototype. Micropumps were implanted subcutaneously in the lumbar region for continuous infusion in the scapular region. Animals received a subcutaneous infusion of the formulation at a dose of 6 mg / kg / day (0.3 mg / kg / hour) of the appropriate apomorphine formulations to animals over a 20 h period / day for 14 days. Blood samples were collected at the following time point:[0126]Day 1 at 0 h, 6 h post Start of Infusion and 20 h post Start of Infusion (SOI),[0127]Day 7 20 h post Start of Infusion (SOI),[0128]Day 14 at 0 h, 20 h post Start of Infusion (SOI).
[0129]Plasma concentrations were determined by HPLC-ESI-MS / MS method and PK parameters were calculated.
[0130]In order to evaluate the local tolerance of the tested formulations, tissue from the injection site was collected from each rat and examined by an anatomo-pathol...
example 6
Local Tolerance in the 14-Day Subcutaneous Infusion Experiment
[0144]The local tolerance data of the tested formulations in subcutaneous infusion over 20 h / day in rats at 6 mg / kg / d are presented in the Table 6. The tolerance scoring was established at the 14th day of infusion, i.e., at the EOI.
TABLE 6Test 1Parameter(Reference)Test 2Test 3Test 4Test 5 Test 6Concentration4.5828.929.630.439.537.2(mg / mL)pH3.36.05.85.95.95.9Tolerance scoring3.02.82.32.72.42.6The same tolerance level than the Apo-Go ® reference (Test 1) was obtained with the test formulations that show a 6 to 8 times higher concentration.
[0145]Thus, using a formulation according to the present invention, patients will be able to receive a better treatment based on a 6 to 8 times lower injection volume, a formulation at a more physiologically acceptable pH and a formulation that tends to be more tolerated than the APO-go® reference.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water miscible | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com