Generation of ctl lines with specificity against multiple tumor antigens or multiple viruses
a technology of ctl lines and tumor antigens, applied in the field of cell biology, immunology, molecular biology, medicine, can solve the problems of generating resistant variants, substantial toxicities, and viral infections accounting for substantial morbidity and mortality in immunocompromised hosts, and achieve the effect of reducing cell death and proliferating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of TAA-Specific CTL from Healthy Donors Using Pepmix-Pulsed or TAA-Expressing as APCs
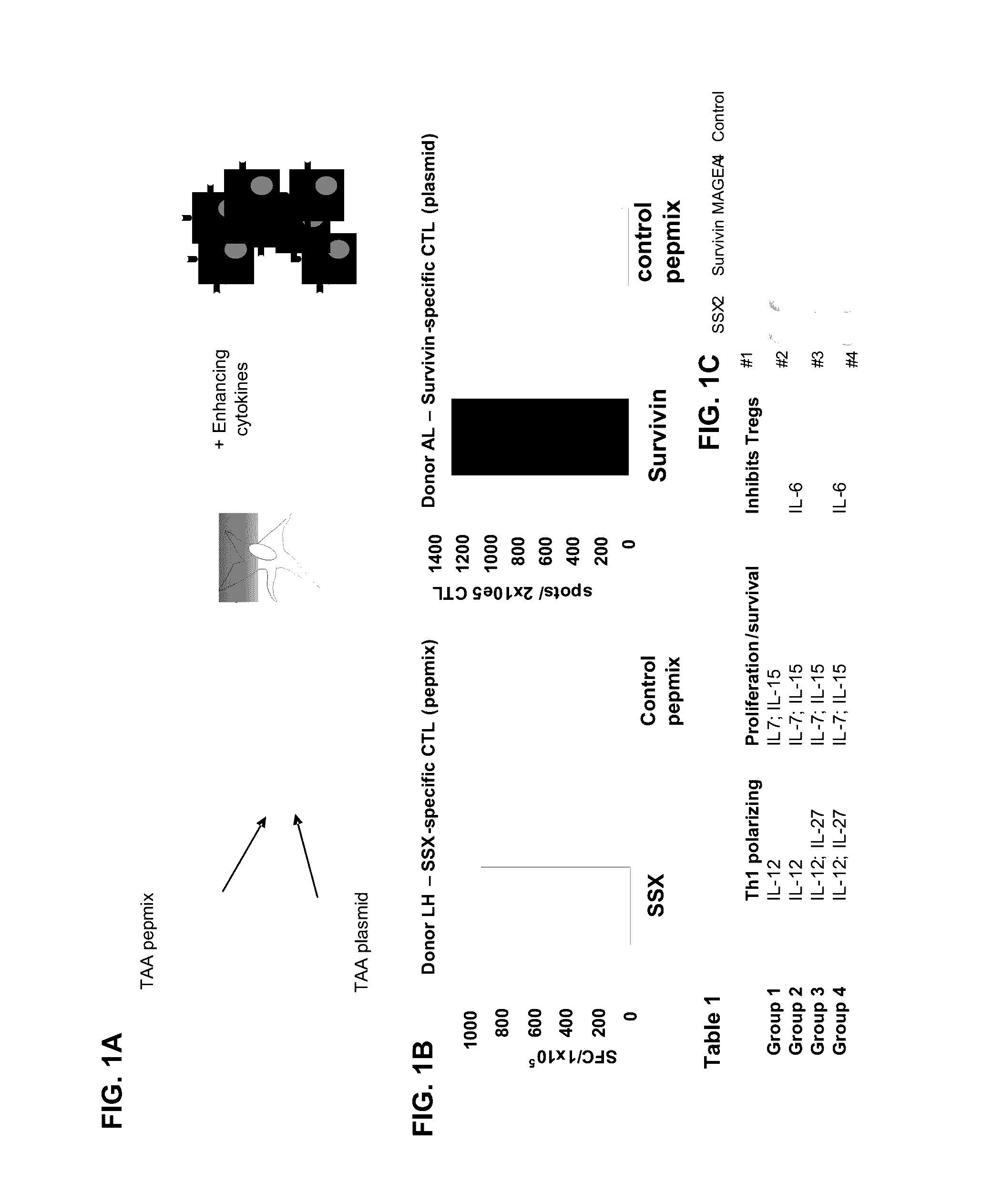
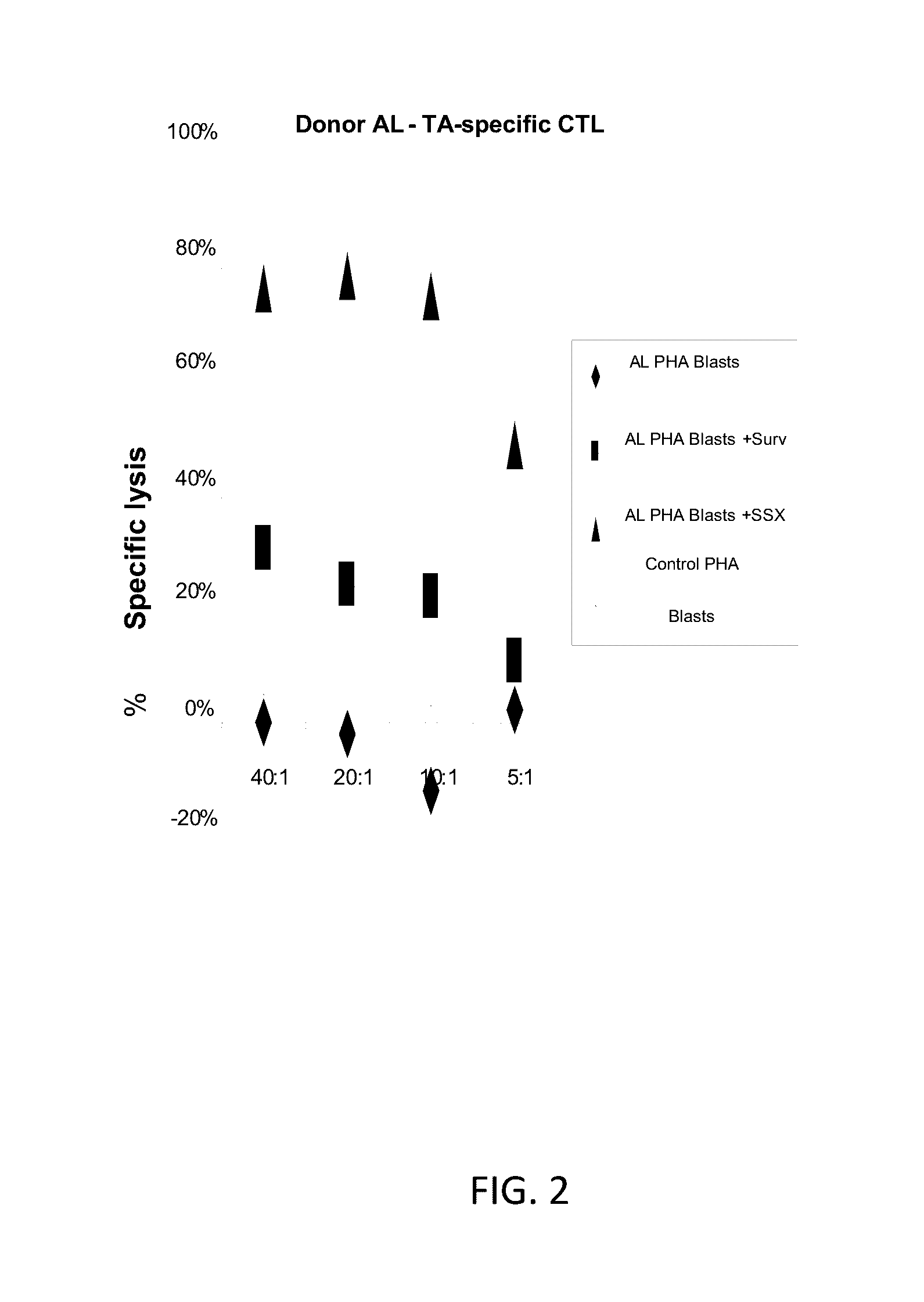
[0242]Production of TA-specific CTL from healthy donors. TAA pepmix-pulsed or plasmid transfected DCs were used to reactivate tumor antigen-specific T cells at a 10:1 ratio (FIG. 1A). PBMCs were cultured in the presence of IL7 (10 ng / ml), IL12 (10 ng / ml) and IL15 (10 ng / ml). A second stimulation on day 9 with plasmid transfected / pepmix-pulsed DCs with addition of IL-7 restimulates the tumor antigen-specific T cells in the culture, which are subsequently fed with IL-2 twice weekly from day 12. The specificity of the expanded TAA-CTL lines was assessed by IFN-γ ELIspot on day 16-17. Results from 2 healthy donors are shown in FIG. 2B; the left panel shows CTL specificity of a line generated using SSX2 pepmix-pulsed DCs as APCs while the right panel shows the specificity of a line generated DCs transfected with a plasmid encoding Survivin as a stimulus. To optimize the CTL generation protocol...
example 2
Exemplary Experimental Design and Methods
[0243]In some embodiments, generation of tumor-specific CTL specific for multiple lymphoma-associated target antigens is provided. One can determine whether TAA-CTL can be expanded from subjects with relapsed / refractory HL for adoptive transfer, either alone or in combination with epigenetic pharmacotherapy.
[0244]These multispecific CTL are more effective in vivo since tumor immune surveillance evasion mechanisms will be minimized, in particular aspects of the invention.
[0245]Regarding exemplary method embodiments, one can compare 2 antigen sources; (i) pepmixes (for example, 15-mers overlapping by 11 aa) spanning the TAAs and (ii) TAA-encoding DNA plasmids, and DCs are APCs for stimulation. Studies showing TAA-CTL generated from healthy donors using both antigen sources are shown in FIG. 1B. For generating multiTAA-CTL one can generate a mastermix of 3 or more pepmixes spanning the most frequently-expressed antigens or one can transfect DCs ...
example 3
Administration of Tumor-Associated Antigen (TAA)-Specific Cytotoxic T-Lymphocytes to Patients with Active or Relapsed Hodgkin's or Non-Hodgkin's Lymphoma
[0259]The present example considers exemplary embodiments for administration of TAA-specific CTLs for individuals with active or relapsed Hodgkin or non-Hodgkin lymphoma, although the skilled artisan recognizes that these practices can be applied to and / or adapted to any other types of cancer.
[0260]In certain embodiments, the present invention concerns injections (such as intravenous) of autologous or allogeneic TAA-specific CTLs in individuals with Hodgkin's (HL) or non-Hodgkin's lymphoma (NHL).
[0261]Immunotherapy Using EBV-Specific T Cells for EBV+Ve HL and NHL
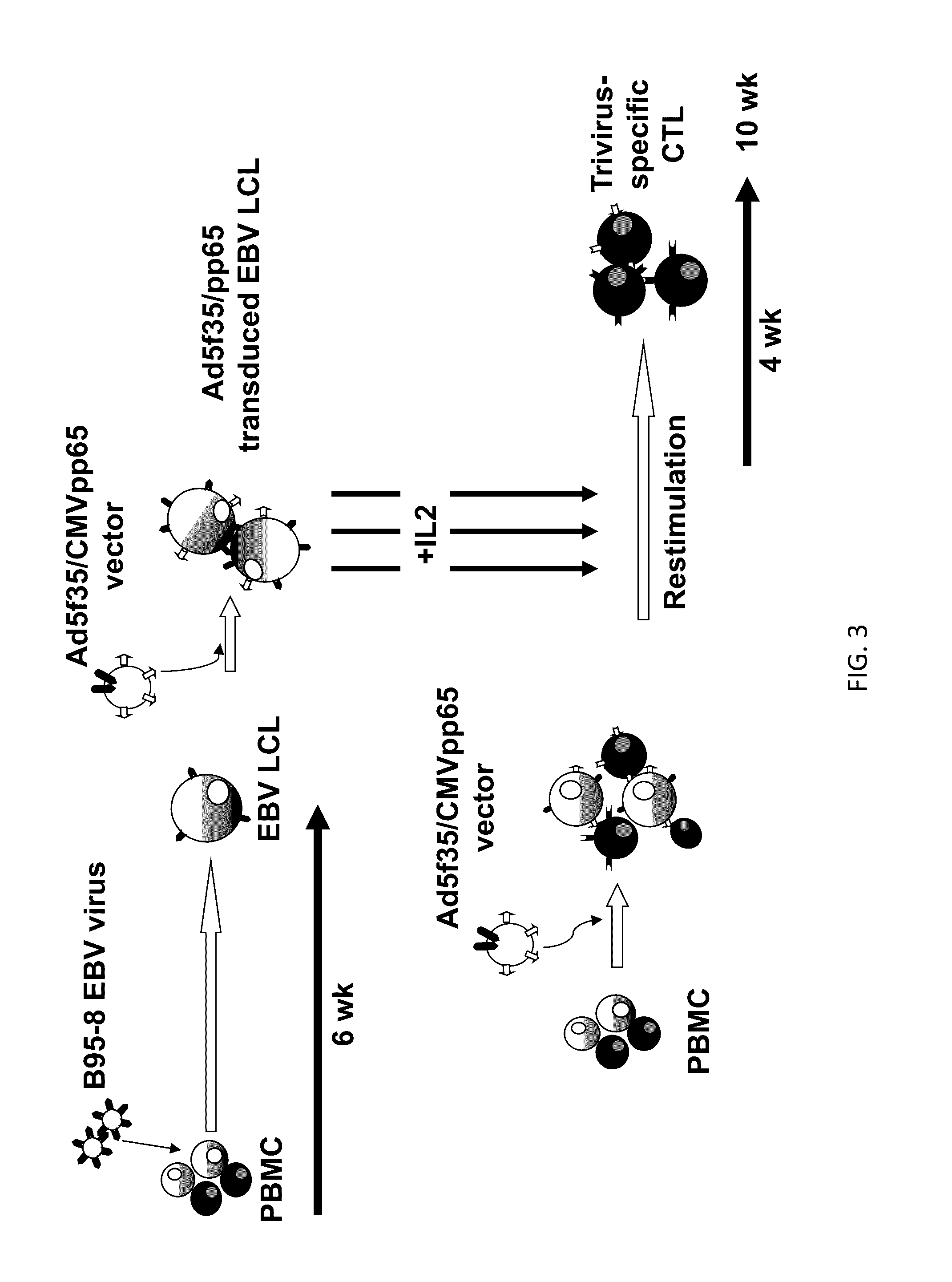
[0262]EBV is associated with HL and NHL in immunocompetent patients and in these cases, the tumor cells express three of about 90 EB viral antigens. To optimize the antigenic targeting of CTLs directed against HL / NHL in immunocompetent subjects, the inventors have prepared C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com