Amorphous solid form of a bet protein inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Amorphous Solid Form of Compound 1-(S)
[0128]
Preparation of Starting Material 2-bromo-1-pyridin-2-ylethanone hydrobromide
[0129]Bromine (Br2, 198 g, 1240 mmol) dissolved in acetic acid (100 mL) was added slowly to a mixture of 1-(pyridin-2-yl)ethanone (150 g, 1238 mmol) in acetic acid (1500 mL) at rt. The red reaction solution was heated to 105° C. for 1 hour to give an off-white slurry. The mixture was stirred for 1 hour at rt, cooled in a water bath (caution acetic acid freezes), filtered, then the solids were washed with acetic acid (150 mL) and ethyl acetate (250 mL). The solid was suspended in acetic acid (1500 mL) and heated to 105° C. for 1 h (note: material did not completely dissolve). The reaction was allowed to cool to rt in a water bath. The precipitate was filtered, washed with acetic acid 100 mL and ethyl acetate 300 mL, and dried under house vacuum at rt to give crude 2-bromo-1-pyridin-2-ylethanone hydrobromide (305 g, 87%) as an off-white powder, which w...
example 2
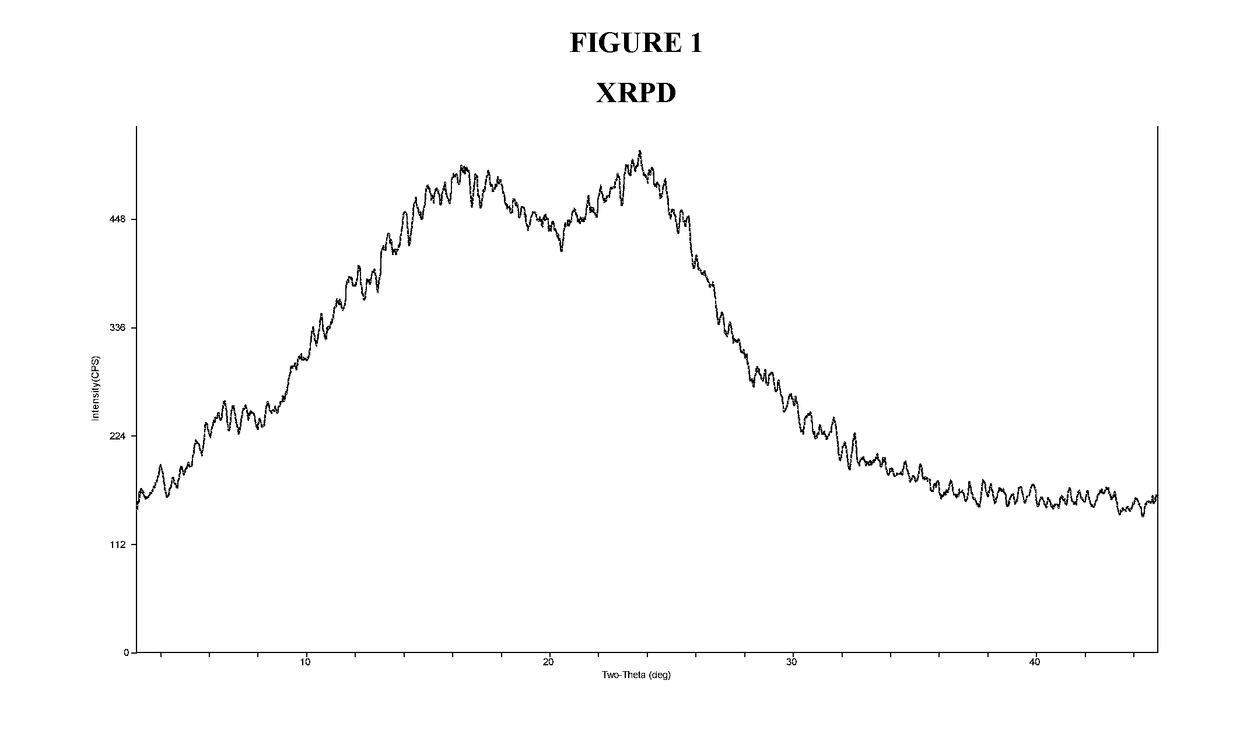
X-Ray Powder Diffraction (XRPD) of Amorphous Compound 1-(S)
[0137]Amorphous Compound 1-(S) was characterized by XRPD. The X-Ray Power Diffraction (XRPD) was obtained from Rigaku MiniFlex X-ray Powder Diffractometer (XRPD). The general experimental procedures for XRPD were: (1) X-ray radiation from copper at 1.054056 Å with Kβ filter; (2) X-ray power at 30 KV, 15 mA; and (3) the sample powder was dispersed on a zero-background sample holder. The general measurement conditions for XRPD were: Start Angle 3 degrees; Stop Angle 45 degrees; Sampling 0.02 degrees; and Scan speed 2 degree / min. The XRPD pattern displays an amorphous halo as shown in FIG. 1.
example 3
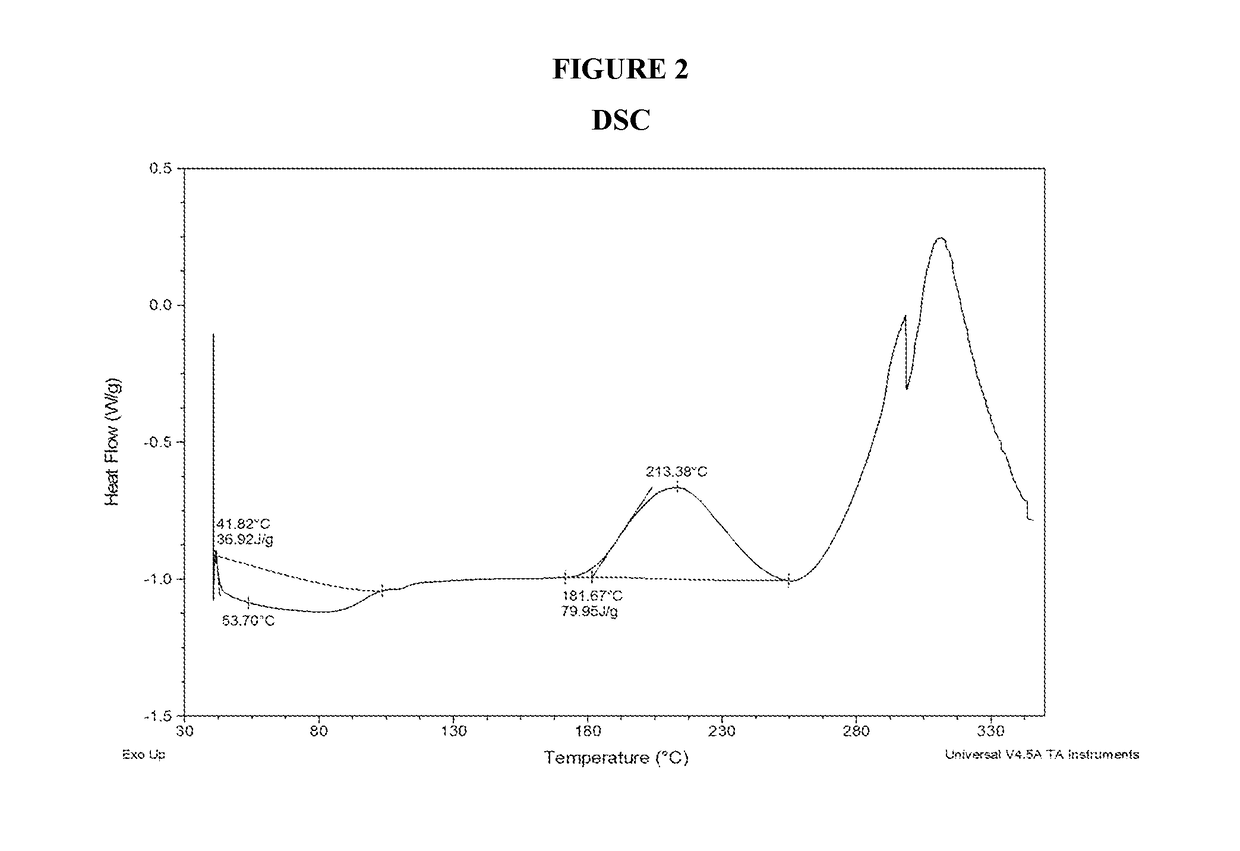
Differential Scanning Calorimetry (DSC) of Amorphous Compound 1-(S)
[0138]Amorphous Compound 1-(S) was characterized by DSC. The DSC was obtained from TA Instruments Differential Scanning calorimetry, Model Q200 with autosampler. The DSC instrument conditions were as follows: 30-350° C. at 10° C / min; Tzero aluminum sample pan and lid; and nitrogen gas flow at 50 mL / min. The DSC thermogram is shown in FIG. 2. The DSC thermogram revealed one exothermic event at an onset temperature of 181.7° C. with a peak temperature of 213.4° C. which corresponds to the decomposition of the compound. The endothermic event below 100° C. is believed to be due to dehydration. No melting point was observed due to the amorphous nature of the compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com