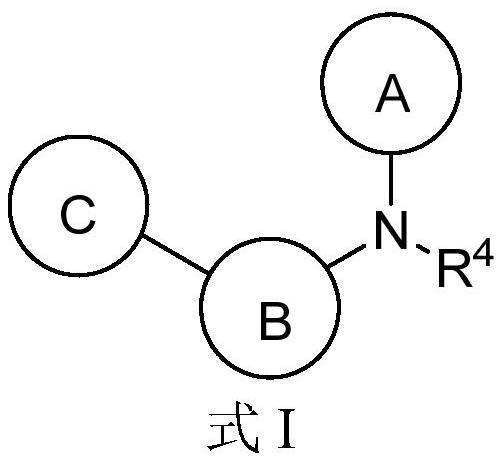
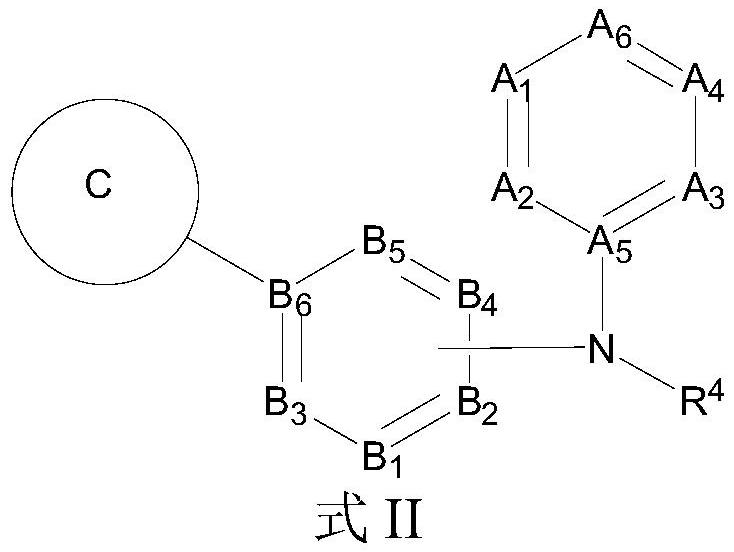
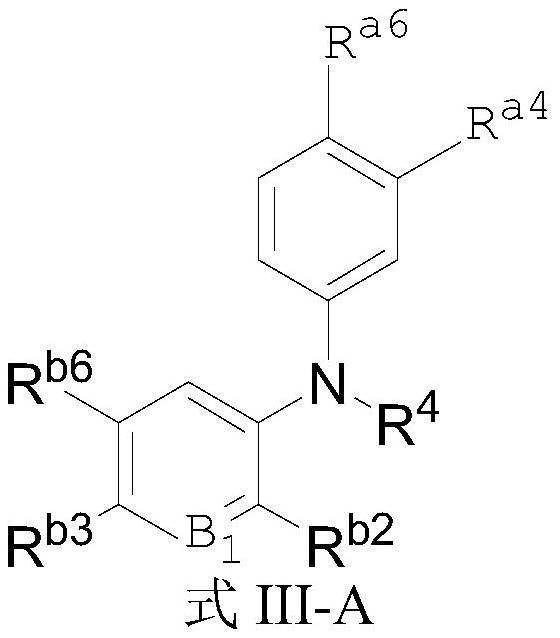
Aromatic amine compound and application thereof in preparation of AR and BRD4 dual inhibitors and regulators
A compound and solvate technology, applied in the field of drug synthesis, can solve problems such as adverse reactions and drug resistance of small molecule drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 Synthesis of N-(3-bromo-4-(1H-imidazol-1-yl)phenyl)-5-(3,5-dimethylisoxazol-4-yl)-N-ethyl- 2-Methylaniline (Compound 31)
[0094]
[0095] first step:
[0096] 2-Bromo-1-fluoro-4-iodobenzene (3.0g, 10mmol), imidazole (680mg, 10mmol) and cesium carbonate (4.9g, 15mmol) were sequentially added to 50mL of DMA, heated to 120°C under nitrogen protection After reacting overnight, TLC monitored the completion of the reaction, cooled naturally to room temperature, extracted with 100mL ethyl acetate, washed with saturated brine (3*50mL), dried over anhydrous sodium sulfate, concentrated, separated and purified by chromatographic column (PE / EA=4 / 1 ) to obtain 3.4 g of compound 1-(2-bromo-4-iodobenzene)-1H-imidazole, yield: 95%.
[0097] Step two:
[0098] (1) Synthetic raw material 5-(3,5-dimethylisoxazol-4-yl)-2-methylaniline (ie compound 31-2): (3,5-dimethylisoxazole- 4-yl)boronic acid (16.9g, 120mmol), 5-bromo-2-methylaniline (22.3g, 120mmol) were dissolved in...
Embodiment 2
[0102] Example 2 Synthesis of 5-(3,5-dimethylisoxazole-4-)-N-ethyl-2-methyl-N-(4-(5-methyl-1H-pyrazole-3- ) phenyl) aniline (compound 97)
[0103]
[0104] first step:
[0105] Dissolve 60% sodium hydride (80mg, 2mmol) in 10ml N,N-dimethylformamide, add 3-(4-bromophenyl)-5-methyl-1H-pyrazole (236mg, 1mmol), room temperature Stir for 10 min, add 2-(trimethylsilyl)ethoxymethyl chloride (182 mg, 1.1 mmol), react at room temperature for 3 hours, wash with 10 ml of water, extract with 10 ml of ethyl acetate, concentrate the organic layer under reduced pressure, and perform thin layer chromatography Separation and purification gave 341 mg of compound 3-(4-bromophenyl)-5-methyl-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazole, yield: 93 %.
[0106] Step two:
[0107] Dissolve 5-(3,5-dimethylisoxazole-4-)-2-methylaniline (202mg, 1mmol) in 5ml 1,4-dioxane, add 3-(4-bromobenzene yl)-5-methyl-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazole (341mg, 0.93mmol), add cesium carbonate...
Embodiment 3
[0112] Example 3 Synthesis of 4-(4-((5-(3,5-dimethylisoxazole-4-)-2-methylphenyl)(ethyl)amino)phenyl)thiazol-2-amine (Compound 98)
[0113]
[0114] first step:
[0115] Dissolve 4-(4-bromophenyl)thiazol-2-amine (254mg, 1mmol) in 10ml of dichloromethane, add phthalic anhydride (140mg, 0.96mmol), add triethylamine (202mg, 2mmo) to reflux Stir for 6 hours, wash with 10 ml of 1N hydrochloric acid, extract with 10 ml of ethyl acetate, wash with 10 ml of saturated sodium bicarbonate, and wash with 10 ml of saturated brine. The organic layer was concentrated under reduced pressure, separated and purified by thin layer chromatography to obtain 360 mg of compound 2-(4-(4-bromophenyl)thiazole-2-)isoindoline-1,3-dione, yield: 94% .
[0116] Step two:
[0117] Dissolve 5-(3,5-dimethylisoxazole-4-)-2-methylaniline (202mg, 1mmol) in 5ml 1,4-dioxane, add 2-(4-(4 -Bromophenyl)thiazole-2-)isoindoline-1,3-dione (360mg, 0.94mmol), cesium carbonate (650mg, 2mmol), BINAP (62mg, 0.1mmol), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com