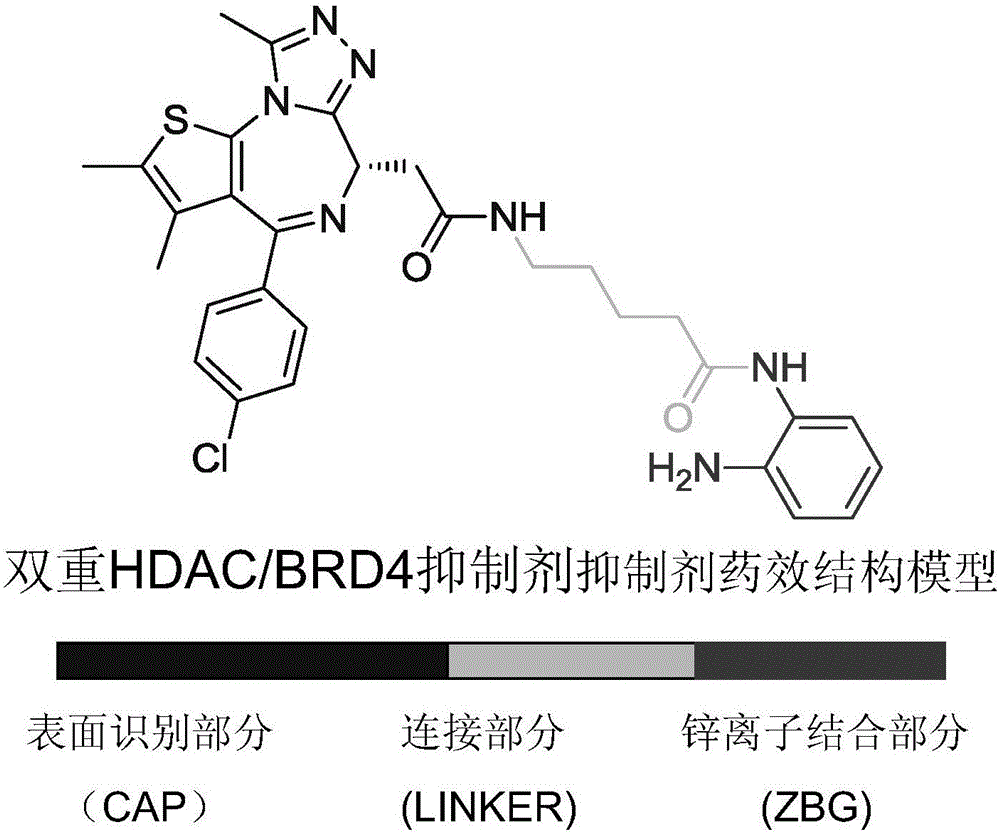
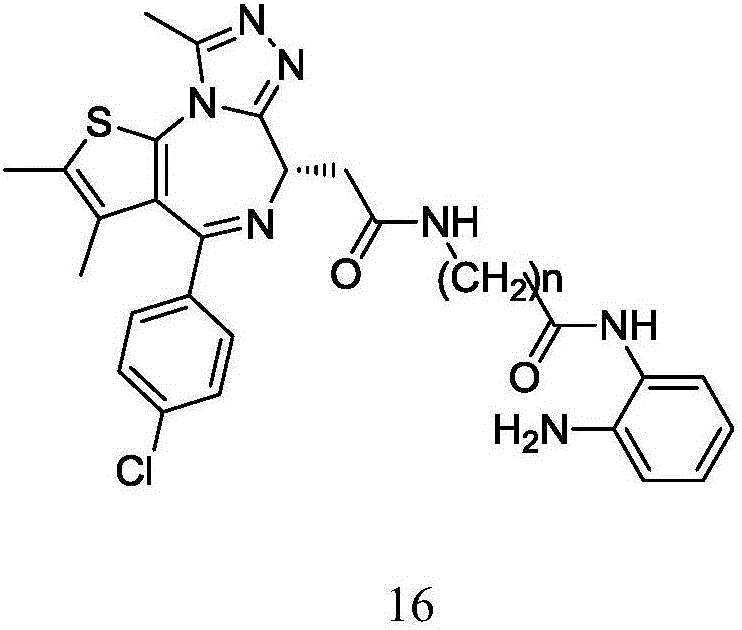
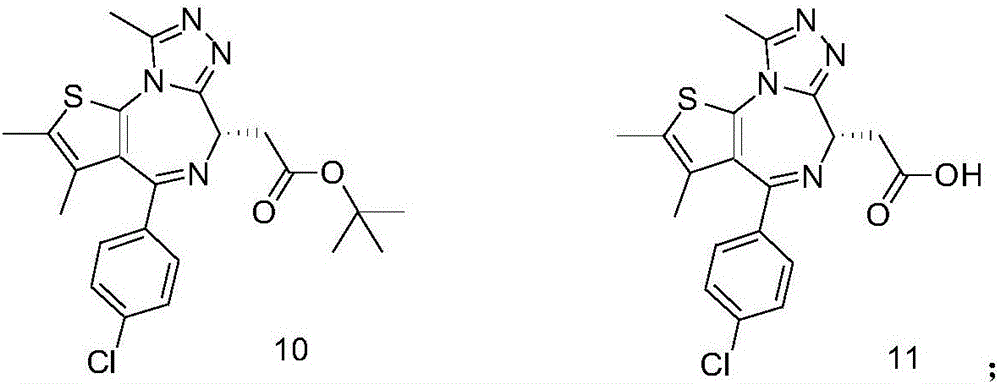
Dual HDAC/BRD4 inhibitor and preparation method and application thereof
An inhibitor, dual technology, applied in the field of medicine and chemical industry, can solve the problems such as no dual HDAC/BET inhibitor reports, achieve high yield, improve therapeutic effect, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of compound 3
[0067]
example 1-1
[0069] Weigh compound 1 (2.85g, 20mmol) and dissolve it in 20mL N,N-dimethylacetamide, add 10mL acetonitrile, 0.1g copper chloride, 3g potassium hydroxide in sequence, and react at room temperature (25°C) under an oxygen atmosphere 12h. After the reaction, 1 mol / L hydrochloric acid was slowly added dropwise to adjust the pH to 7, filtered through diatomaceous earth, the filtrate was washed three times with water, and then washed three times with saturated brine, and the organic layer was dried with anhydrous sodium sulfate and evaporated to dryness. Column chromatography gave a yellow solid, the target compound 3 (2.92 g, yield 82%).
[0070] The target product compound 3 1 The data of H NMR are as follows:
[0071] 1 H NMR (300MHz, DMSO-d6)δ:
[0072] 2.95(s, 2H), 7.58(d, J=8.30Hz, 2H), 7.80(t, J=8.52Hz, 2H)
example 1-2
[0074] Compound 1 (2.85 g, 20 mmol) was weighed and dissolved in 20 mL of tetrahydrofuran, 10 mL of acetonitrile, 0.1 g of copper chloride, and 3 g of potassium hydroxide were added in sequence, and reacted at room temperature under an oxygen atmosphere for 12 h. After the reaction, 1 mol / L hydrochloric acid was slowly added dropwise to adjust the pH to 7, filtered through diatomaceous earth, the filtrate was washed three times with water, and then washed three times with saturated brine, and the organic layer was dried with anhydrous sodium sulfate and evaporated to dryness. Column chromatography gave a yellow solid, the target compound 3 (2.5 g, yield 70%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com