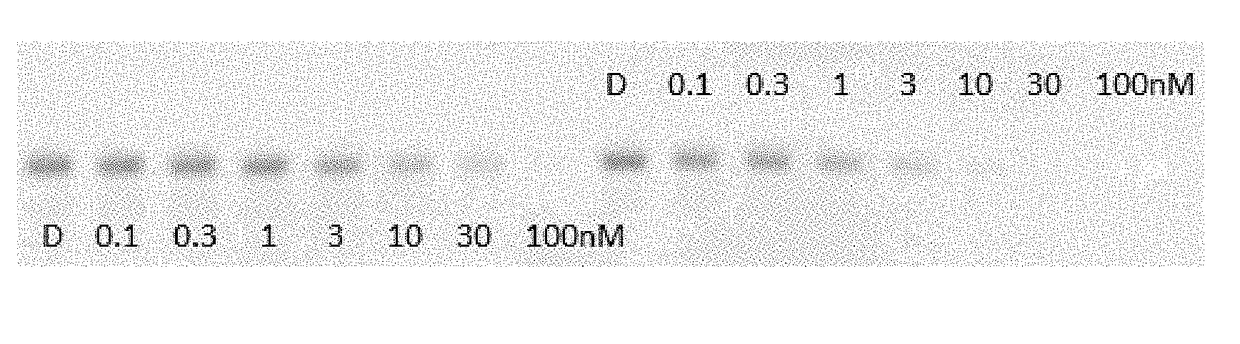
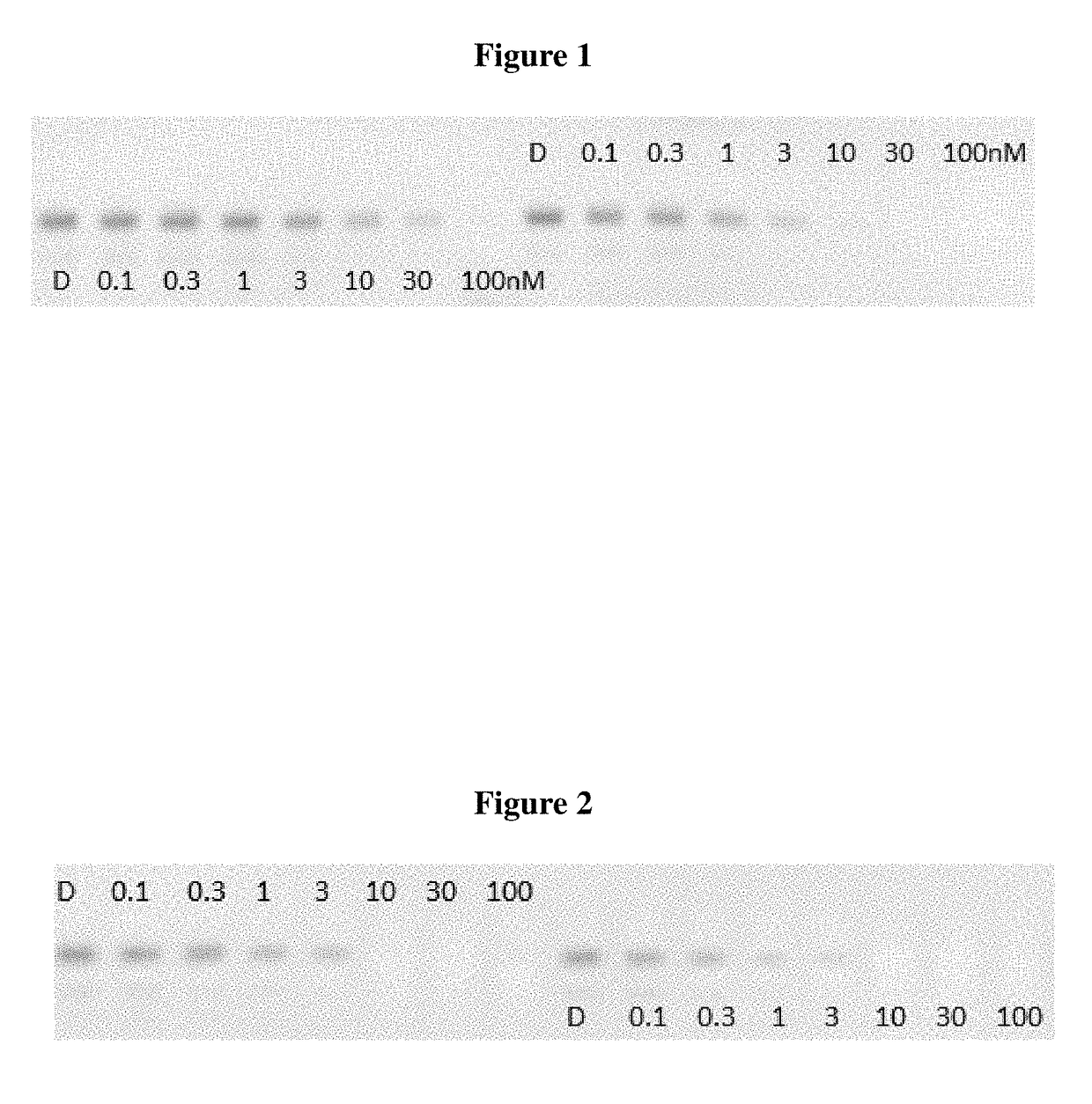
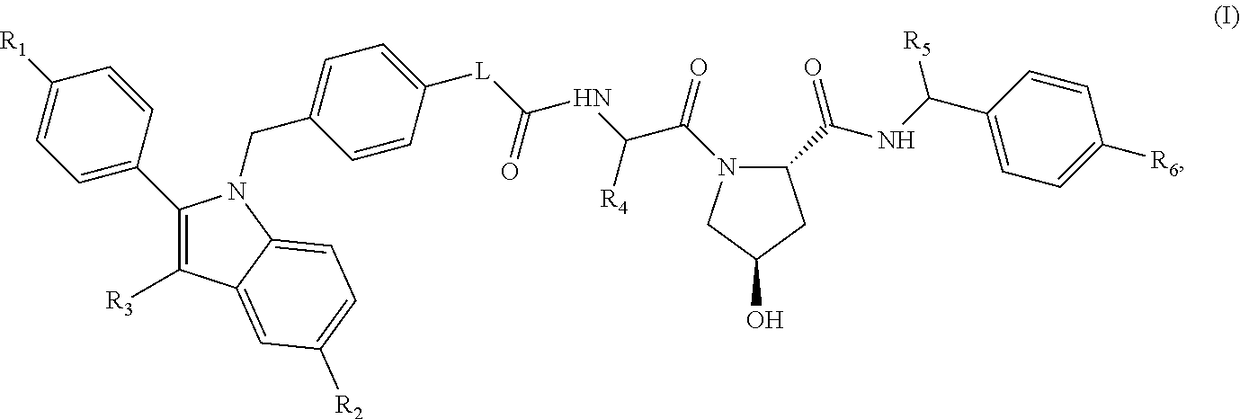
Indole derivatives as estrogen receptor degraders
a technology of estrogen receptor and indole derivatives, which is applied in the field of compounds, compositions, and medicaments, can solve problems such as incomplete blockade of estrogen-mediated activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
Example #1: (2S,4R)-1-[(2S)-2-[1-(4-[[2-(4-fluorophenyl)-5-hydroxy-3-methyl-1H-indol-1-yl]methyl]phenyl)-1,4,7,10-tetraoxadodecan-12-amido]-3,3-dimethylbutanoyl]-4-hydroxy-N-[[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl]pyrrolidine-2-carboxamide
[0409]
Step 1: Preparation of 2-bromo-1-(4-fluorophenyl) propan-1-one
[0410]In a 250 mL round bottom flask, 1-(4-fluorophenyl) propan-1-one (5.0 g, 32.86 mmol, 1.00 equiv) and PyBr3.HBr (11.5 g, 35.96 mmol, 1.10 equiv) were dissolved in dichloromethane (50 mL) at room temperature. The resulting solution was stirred for 1 hour at room temperature. The reaction was then quenched by the addition of water. The resulting mixture was extracted with ethyl acetate (100 mL×2) and the organic layers were combined, washed with brine and dried over anhydrous sodium sulfate. The organic solvent was removed under reduced pressure. This resulted in 5.0 g (66%) of 2-bromo-1-(4-fluorophenyl)propan-1-one as light yellow oil.
Step 2: Preparation of 5-(benzyloxy)-2-...
example # 2
Example #2: (2S,4R)-1-[(2S)-2-[1-(4-[[2-(4-fluorophenyl)-5-hydroxy-3-methyl-1H-indol-1-yl]methyl]phenyl)-1,4,7,10-tetraoxadodecan-12-amido]-3,3-dimethylbutanoyl]-4-hydroxy-N-[(1S)-1-[4-(4-methyl-1,3-thiazol-5-yl)phenyl]ethyl]pyrrolidine-2-carboxamide
[0420]
[0421]In a 50 mL round-bottom flask, 1-(4-[[2-(4-fluorophenyl)-5-hydroxy-3-methyl-1H-indol-1-yl]methyl]phenyl)-1,4,7,10-tetraoxadodecan-12-oic acid (80.0 mg, 0.15 mmol, 1.00 equiv), (2S,4R)-1-[(2S)-2-amino-3,3-dimethylbutanoyl]-4-hydroxy-N-[(1S)-1-[4-(4-methyl-1,3-thiazol-5-yl)phenyl]ethyl]pyrrolidine-2-carboxamide (66.0 mg, 0.15 mmol, 1.00 equiv), (benzotriazole-1-yloxy)-tris-(dimethylamino)phosphonium hexafluorophosphate (79.0 mg, 1.20 equiv) and N,N-diisopropylethylamine (58.0 mg, 0.45 mmol, 3.00 equiv) were dissolved in N,N-dimethylformamide (2 mL) at 0° C. The resulting solution was stirred for 1 hour at 0° C. The reaction was then quenched by the addition of water. The resulting mixture was extracted with ethyl acetate (20 mL...
example # 3
Example #3: (2S,4R)-1-[(2S)-2-[2-[2-([1-[2-(4-[[2-(4-fluorophenyl)-5-hydroxy-3-methyl-1H-indol-1-yl]methyl]phenoxy)ethyl]piperidin-4-yl]oxy)ethoxy]acetamido]-3,3-dimethylbutanoyl]-4-hydroxy-N-[[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl]pyrrolidine-2-carboxamide
[0422]
Step 1: Preparation of 1-benzyl-4-[2-(oxan-2-yloxy)ethoxy]piperidine
[0423]In 250 mL round bottom flask, sodium hydride (5.0 g, 208.33 mmol, 4.00 equiv) was added to a solution of 1-benzylpiperidin-4-ol (9.0 g, 47.05 mmol, 1.50 equiv) in N, N-dimethylformamide (150 mL) at room temperature. The resulting mixture was stirred for 20 minutes at room temperature. Then 2-(2-bromoethoxy)oxane (6.5 g, 31.09 mmol, 1.00 equiv) was added and the reaction mixture was heated to 50° C. and stirred overnight. The reaction was then quenched by the addition of water. The resulting mixture was extracted with ethyl acetate (100 mL×2) and the organic layers were combined, washed with brine and dried over anhydrous sodium sulfate. The organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com