Block co-poly(metal organic nanostructures) (bcpmons) and uses thereof
a technology of metal organic nanostructures and co-polymers, which is applied in the field of block copoly (metal organic nanostructures) (bcpmons), can solve the problems of more difficult to achieve in synthetic polymers, and achieve the effect of low molecular weigh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on and Characterization of BCPMONs of Formula (A) or (B)
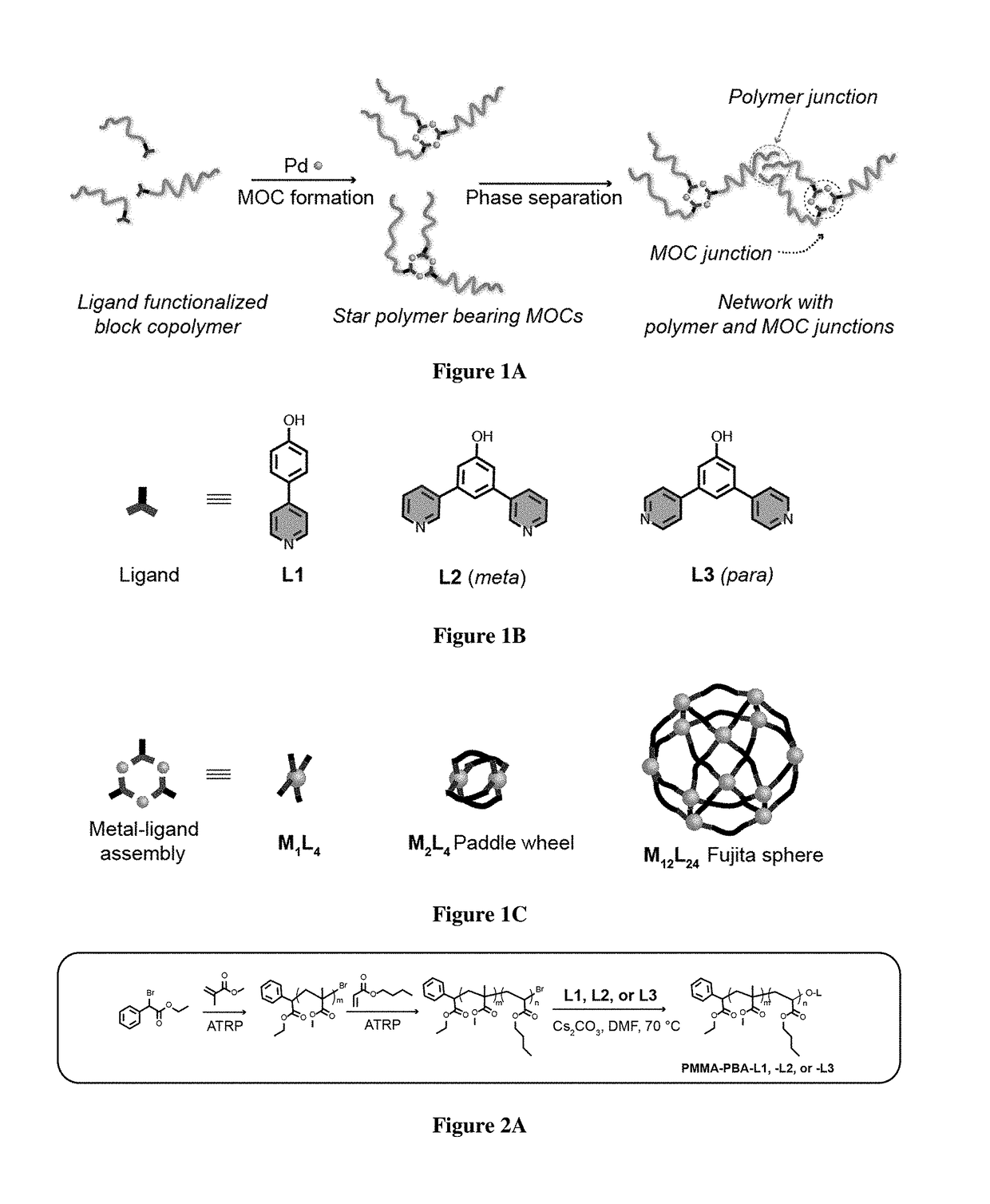
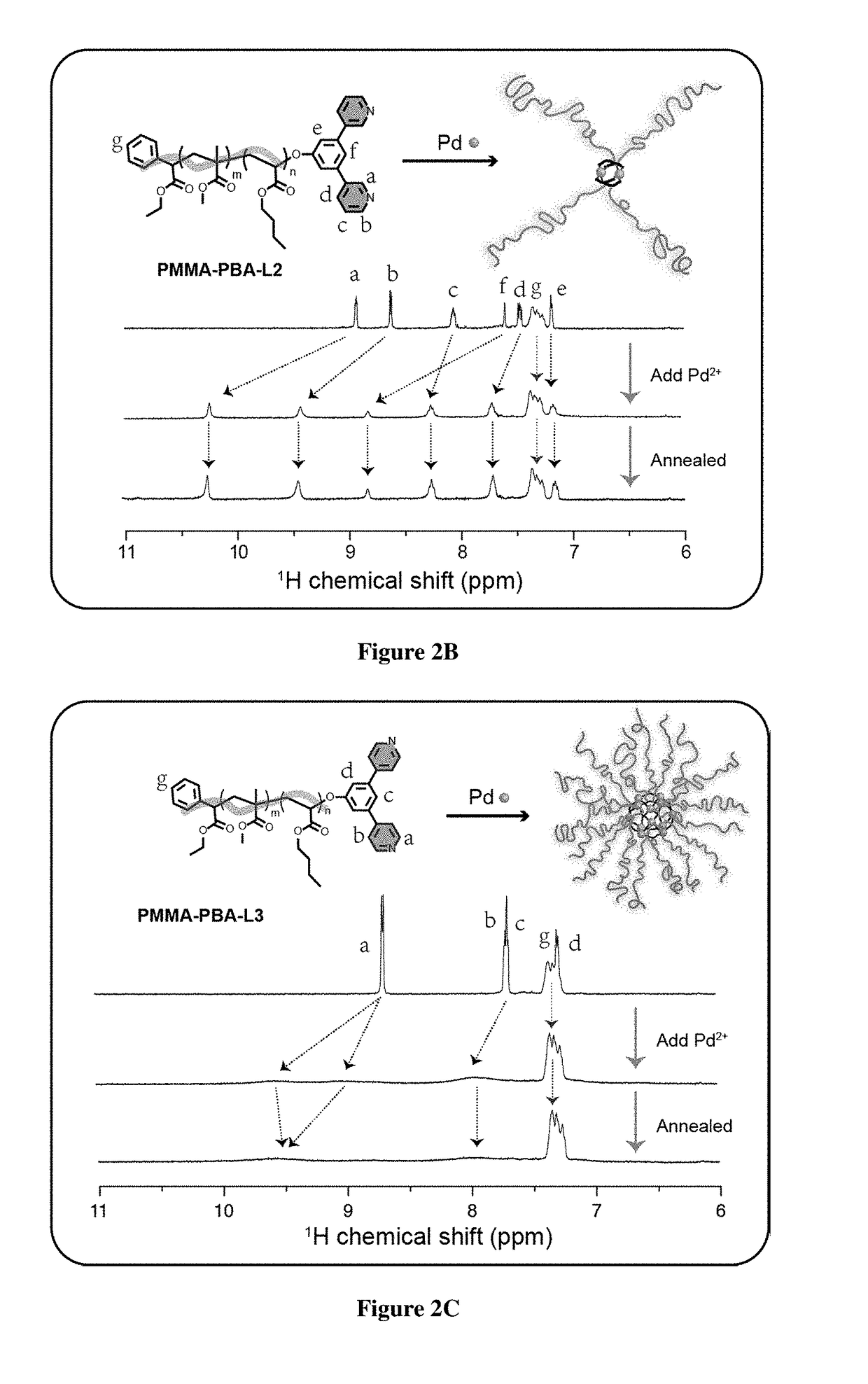
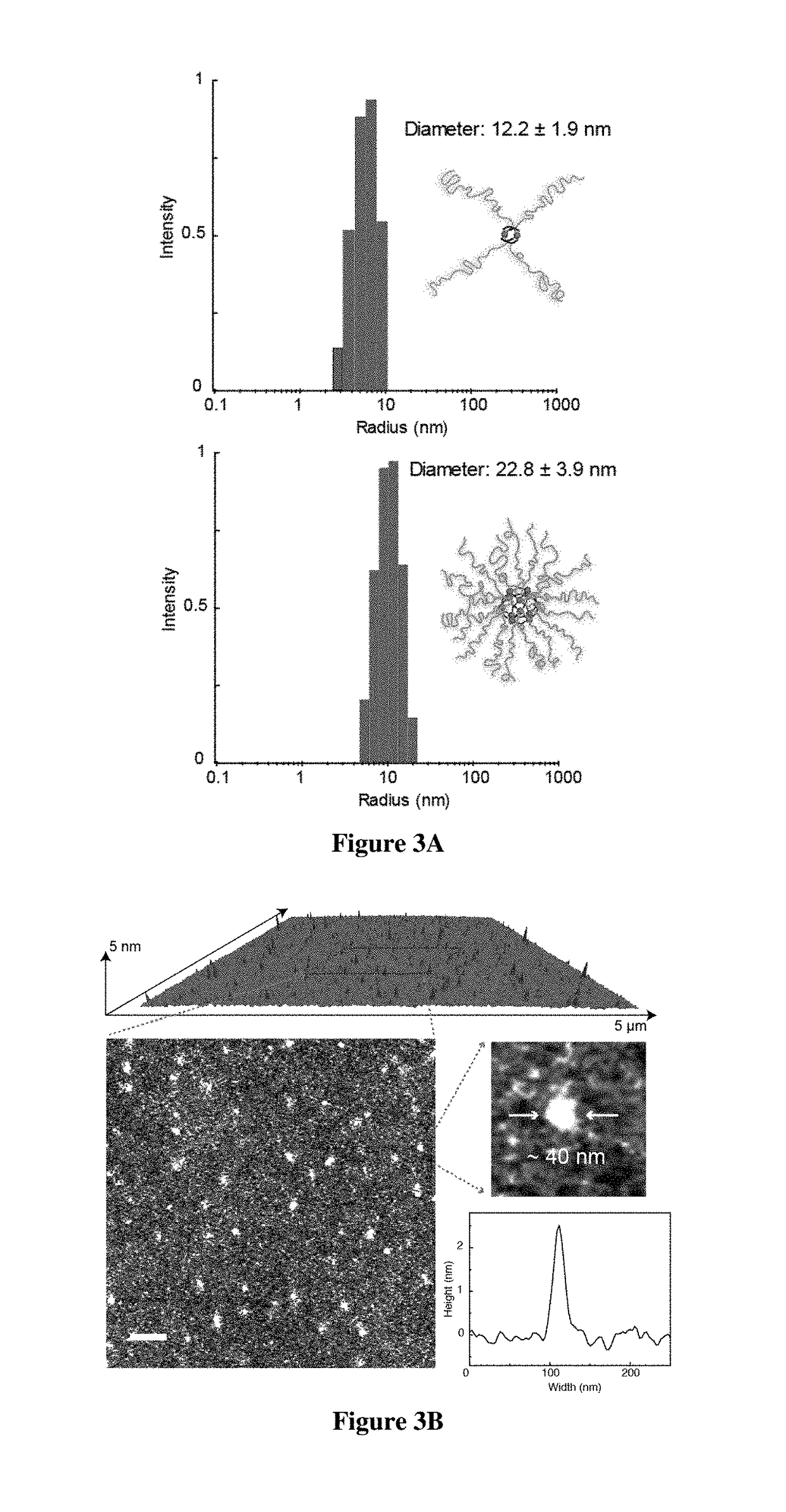
[0746]For the realization of exemplary BCPMONs, poly(methyl methacrylate)-block-poly(n-butyl acrylate) (PMMA-b-PBA, or PMMA-PBA) bearing a pyridyl ligand at the chain end was used. PMMA-PBA is known to phase separate in the bulk state as well as in suitable solvents.56-57 The ligands of choice are shown in FIG. 1B. In the presence of Pd ions, these ligands form PdxLy complexes with different geometries: ligand L1 forms a square planar ML4 complex (this ligand may serve as a control for comparison to BCPMONs), while ligands L2 and L3 are structural isomers of meta- and para-bispyridine that assemble into M2L4 paddlewheel and M12L24 Fujita sphere MONs, respectively. Schematics for these complexes are shown in FIG. 1C.
BCP Synthesis
[0747]The BCPs were synthesized via atom transfer radical polymerization (ATRP), followed by post-polymerization functionalization (FIG. 2A). Using Ethyl a-bromophenylacetate (EBPA) as the initiator, the...
example 2
on and Characterization of BCPMONs of Formula (C)
[0861]Herein, the synthesis of uniform oligomeric polyMOF ligands with alkyne end groups via an iterative exponential growth (IEG) strategy is reported. These ligands were used to prepare a diblock copolymer via copper-catalyzed azide-alkyne cycloaddition “click” chemistry with azide-terminated polystyrene (PS). In the presence of Zn ions, this novel block copolymer forms a BCPMON comprised of crystalline polyMOF domains embedded in an amorphous PS matrix.
[0862]Block copolymer (BCP) assemblies and metal-organic frameworks (MOFs) are two classes of self-assembled matter with vastly different structures and properties. The former may be derived from covalently linked, flexible polymer chains that may undergo phase separation on length scales typically ranging from ˜10-100 nm.1,2 BCPs may be useful for separations, micropatterning, battery, and electronics technologies.1,2 On the other hand, MOFs are crystalline networks, optionally with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com