Inhibition of Platelet Absorption
a platelet absorption and inhibition technology, applied in the field of electrospun coatings, can solve the problems of reducing the potential for activating platelets to be removed, reducing the flow and thrombosis of the graft, and vascular grafting synthesized grafts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
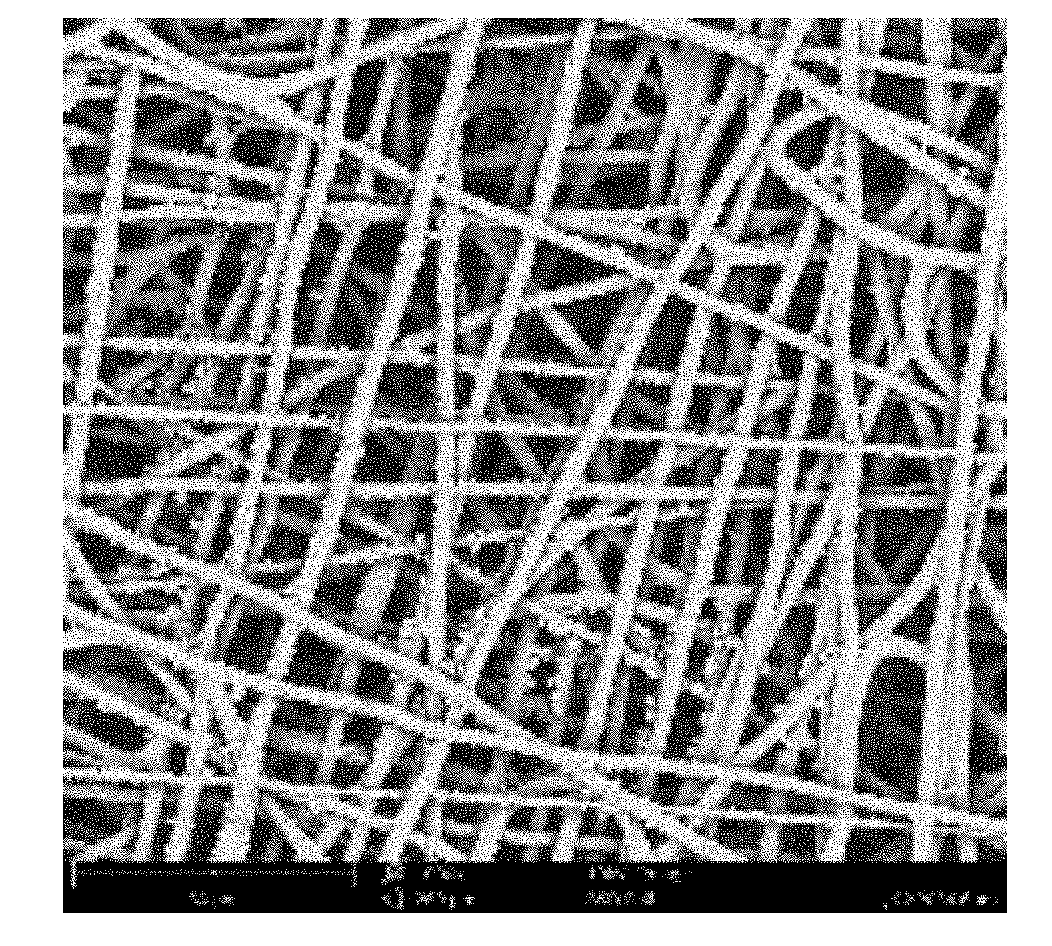
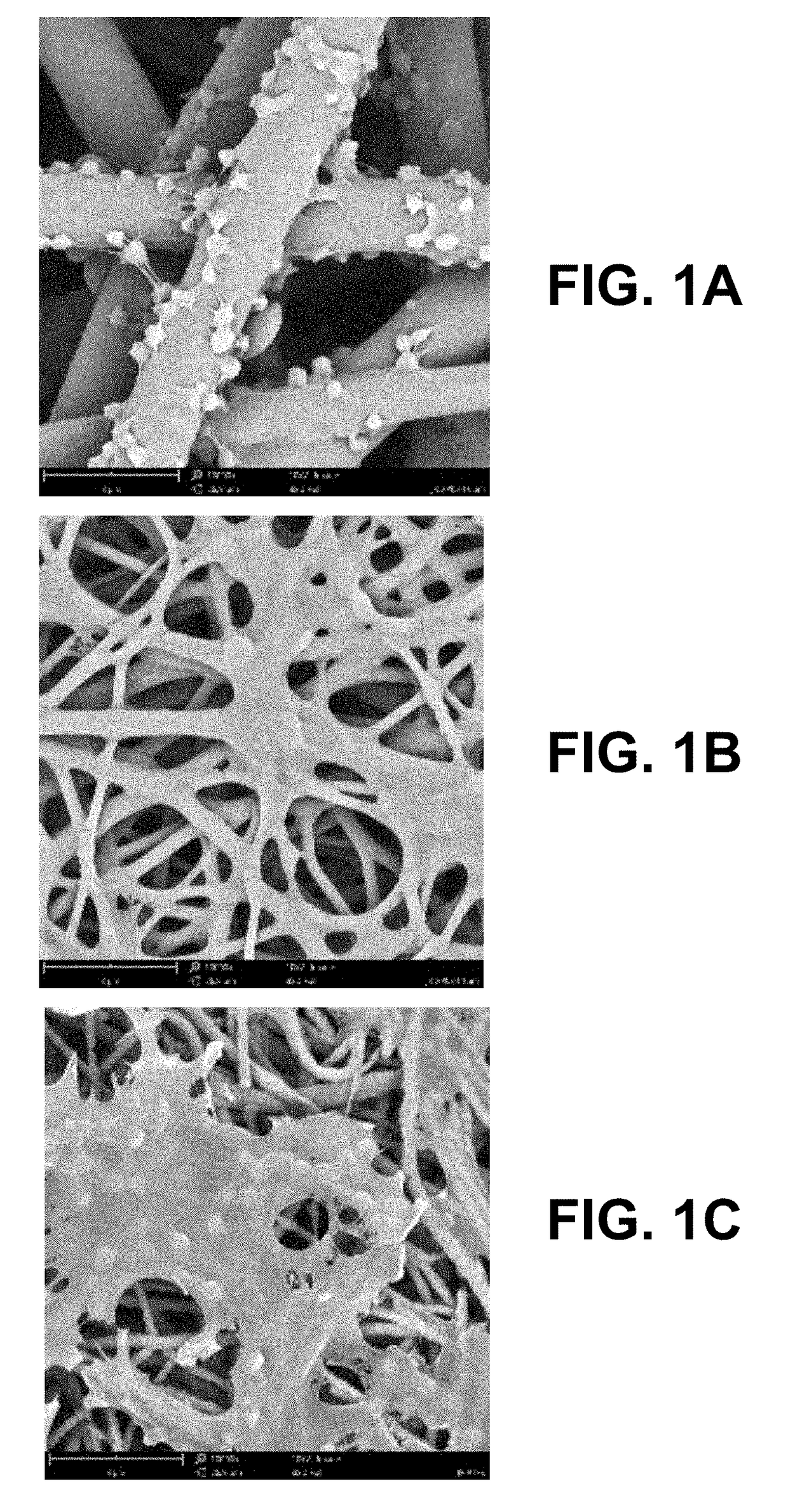
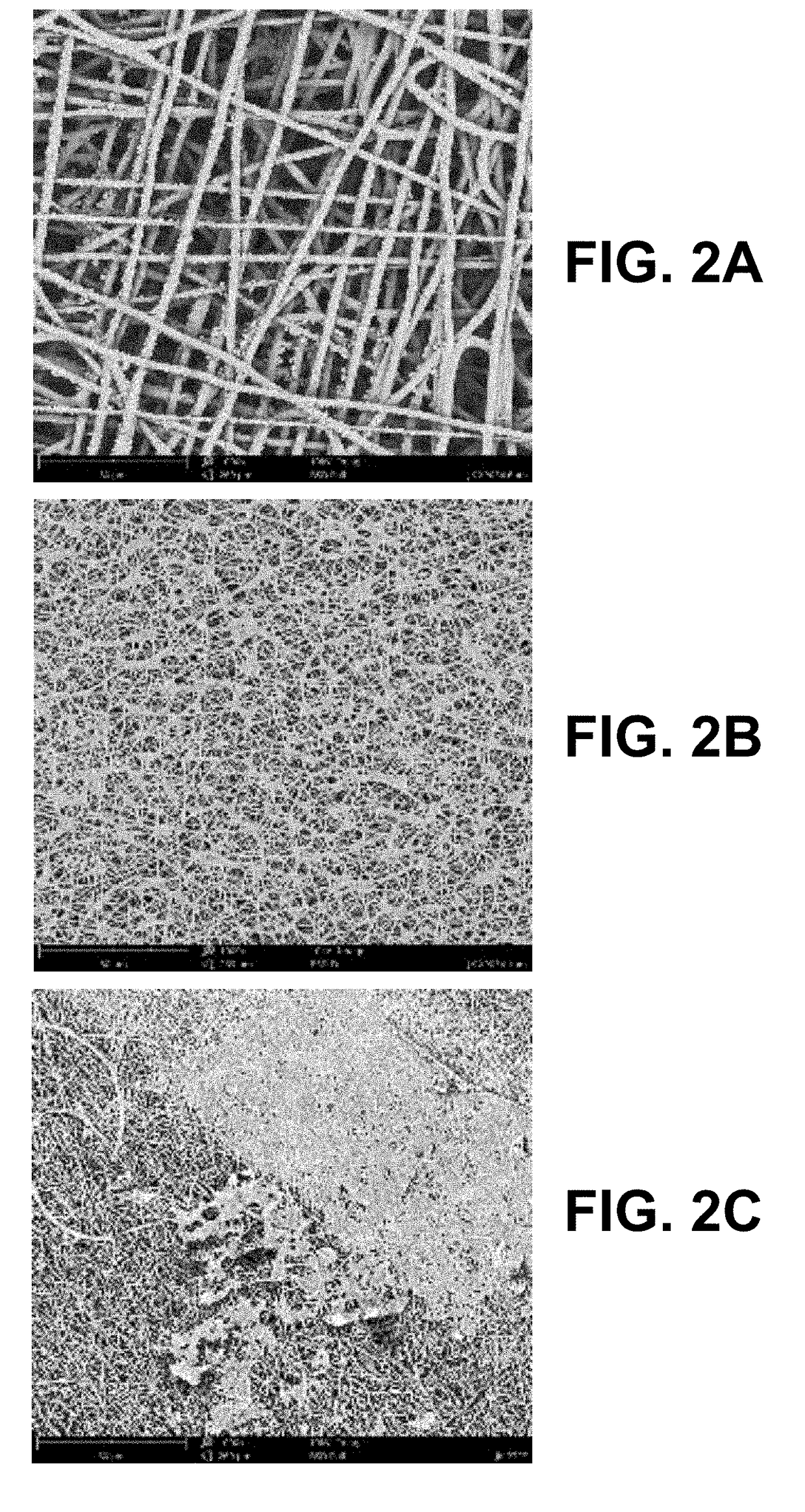
[0031]The invention relates to a cardiovascular graft with highly reduced thrombogenicity by having an electrospun mesh produced from supramolecular polymers (SP). Preferably, the vascular graft is a non-woven mesh and / or large diameter fibers. The invention also relates to a method to produce such grafts via electrospinning. The invention further relates to the implantation of the vascular graft into the human body to allow vascular bypass / reconstruction, or repeated venous access for dialysis treatment, as well as other disorders of small-diameter blood vessels.
[0032]With the design of the cardiovascular graft, as defined infra, the inventors have demonstrated and describe herein unexpected platelet behavior on vascular grafts produced from SP. Platelet activation and adhesion were observed without significant spreading, aggregation or philopodia formation, in stark contrast with widely available “biocompatible” materials such as PTFE. Such outcomes prove that the material is idea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com