EFFLUENT TREATMENT PROCESS - pH REFINEMENT FOR SULPHATE REMOVAL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0031]This example illustrates the effect of pH on the formation of aluminium precipitates.
[0032]The precipitation of various aluminium phases, namely aluminium trihydroxide (Al(OH)3), from sulphate media at pH values of 6.5, 7.0, 7.5, 8.0 and 8.5 was investigated. The effect of variations in pH on the types of solid phases formed was examined. The sulphate medium used consisted of aluminium sulphate solutions (Al2(SO4)3) prepared at 10 g / L. The pH of the medium was controlled with the addition of a caustic soda (NaOH) solution at a concentration of 500 g / L. Results from the precipitation tests revealed that the precipitated phases contained, in addition to aluminium, high amounts of sulphates. This indicated the formation of two phases, namely aluminium trihydroxide (Al(OH)3) and basic aluminium sulphate with the general formula (Al(OH)x(SO4)y). It was also found that the optimum pH for the formation of Al(OH)3 is in the range of 8.0 to 8.5. At this pH, the amount of basic aluminiu...
example 2
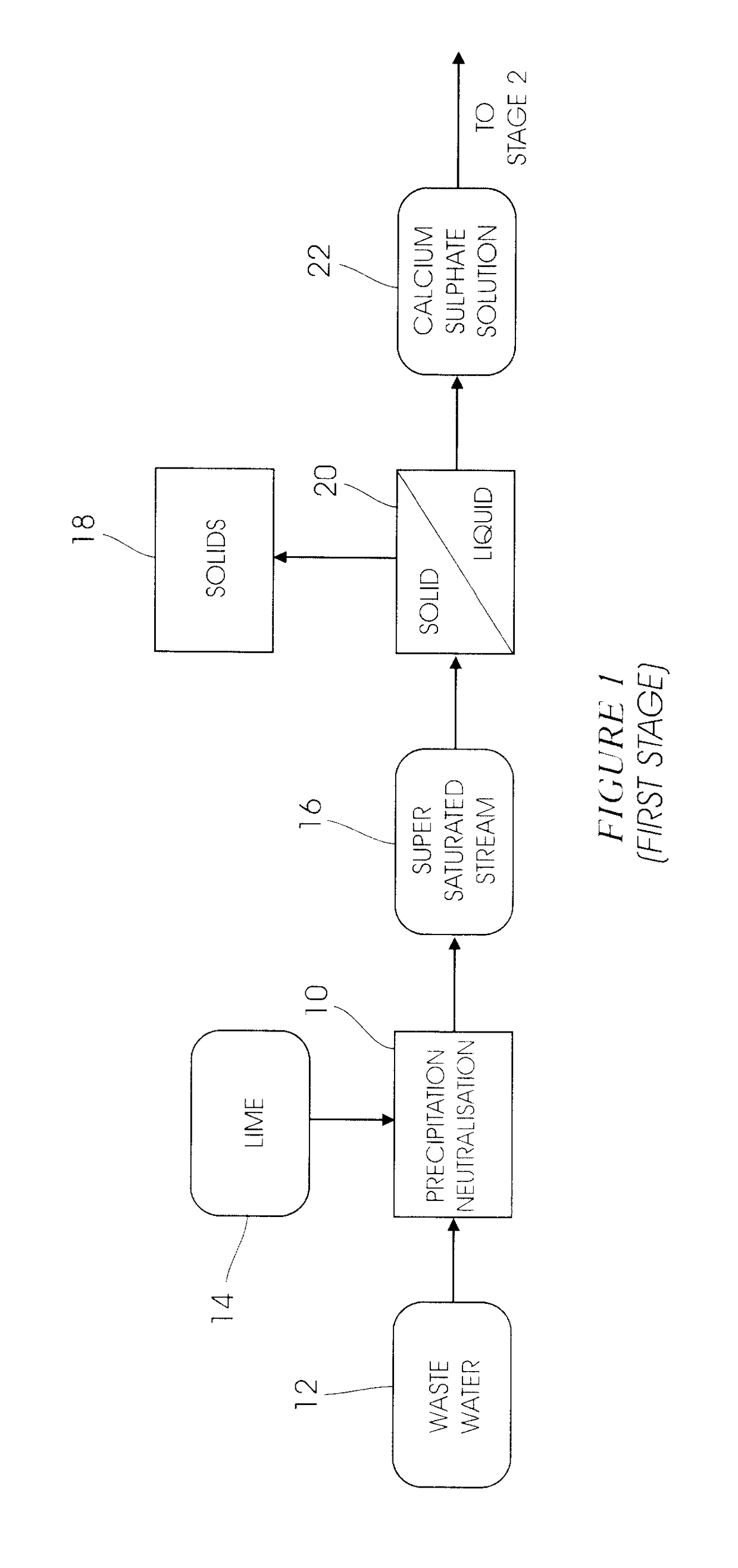
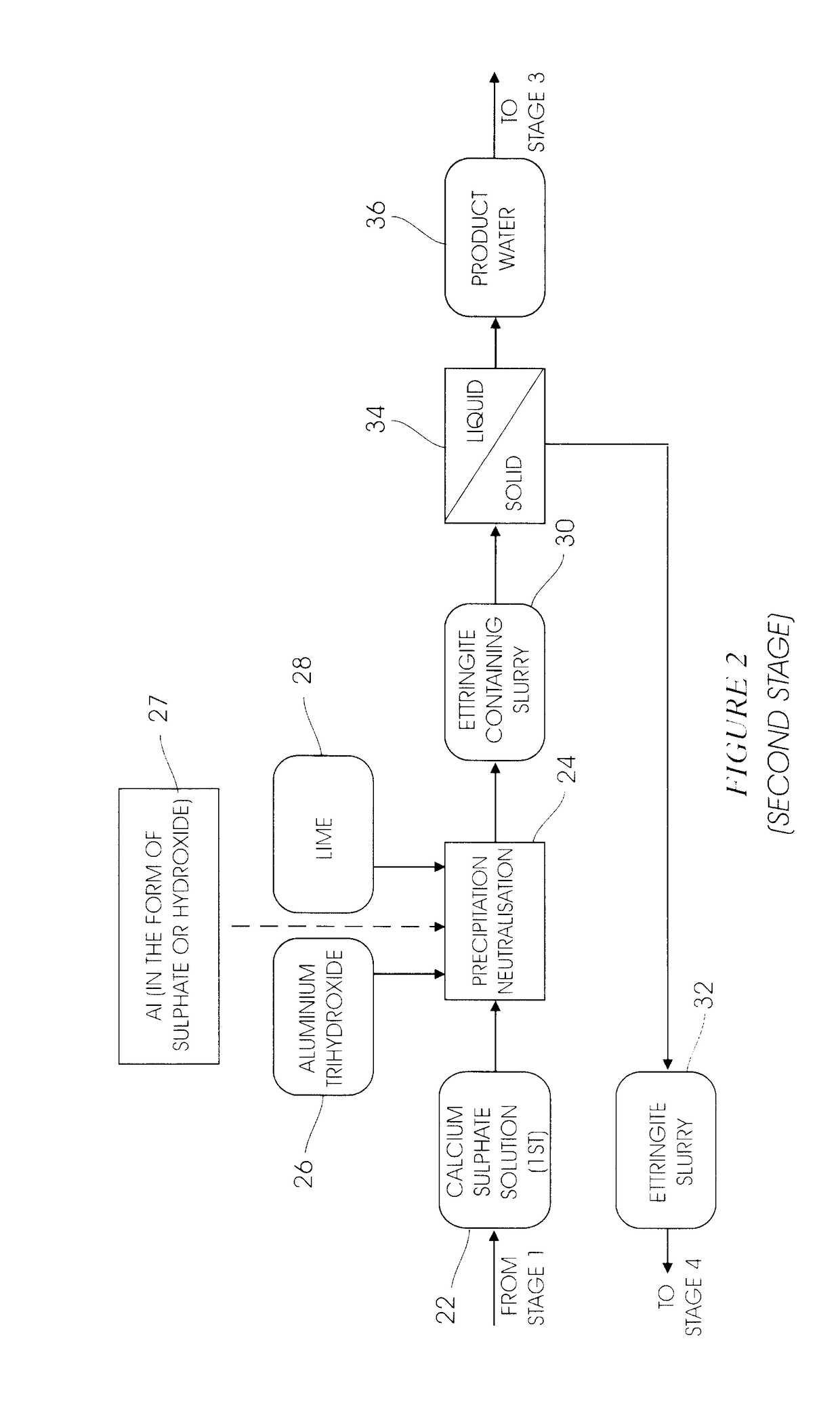
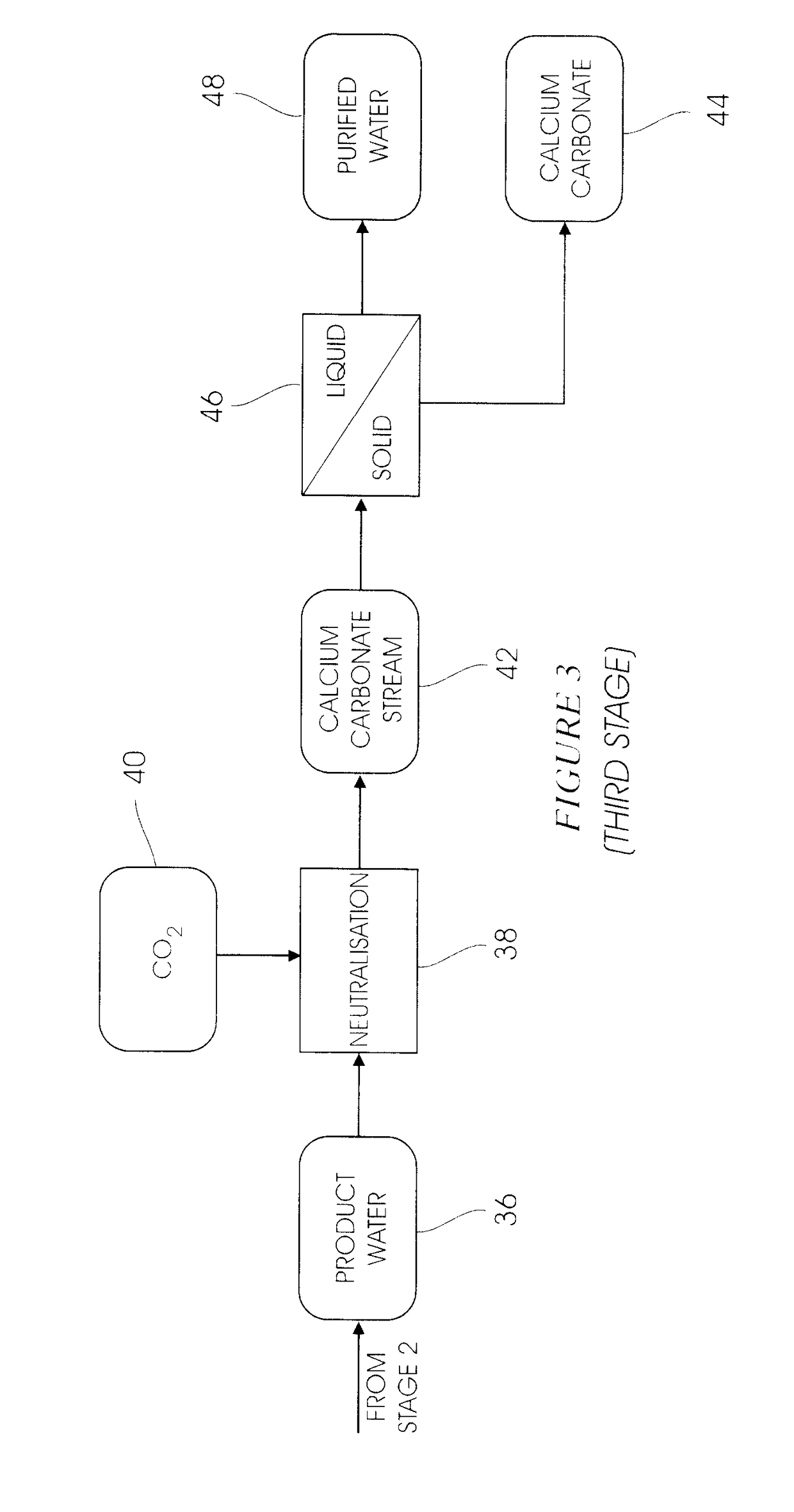
[0033]A fully integrated pilot plant operated as per the diagrams of the type shown in FIGS. 1 to 4 capable of processing 10 L / h of water, was operated for a period of 2 weeks. The combination of the heavy metal precipitation stage and the gypsum de-supersaturation stage was successful and average precipitation efficiencies of 98%, 97%, 96%, 96% and 25% were achieved for magnesium, manganese, aluminium, iron and sulphate respectively. The results in the ettringite precipitation stage showed that the target sulphate concentration of 400 mg / L (SANS Class I specification) in the overflow was reached, and potable water was produced after the carbonation stage in FIG. 3. The results from the ettringite decomposition stage showed a 99.5% recovery of amorphous aluminium trihydroxide precipitate.
example 3
[0034]This example illustrates heavy metal and gypsum precipitation, ettringite precipitation and ettringite decomposition steps of the invention.
[0035]A mini pilot plant capable of processing 100 L / h of acid mine water using the consolidated process of FIG. 1-4, was operated continuously for a period of four weeks. The feed to the plant consisted of a synthetic solution containing bivalent cations such as Mg2+, Ca2+, Mn2+, as well as SO42− and Fe2+. The average feed composition is presented in Table 2.
TABLE 2Feed water composition (expressed in mg / L)MgAlSiCaTiCrMn674262952239CoNiCuZnPbFeSO42−41308
[0036]The results of the pilot campaign showed that the process was effective at removing heavy metals from contaminated water. The treated water produced was nearly free of heavy metal ions, namely iron, aluminium, manganese and magnesium. Removal efficiencies of 97% and 93% were obtained for magnesium and manganese, respectively. Lime consumption was averaged at 1.4 kg / m3 of feed water.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com