Compositions comprising aav expressing dual antibody constructs and uses thereof
a technology of dual antibody and composition, which is applied in the field of compositions comprising aav expressing dual antibody constructs, can solve the problems of inconvenient re-administration, limited duration of efficacy per administration, and inconsistent serum levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Vectors Containing Full-Length Antibody Co-Expression Cassettes
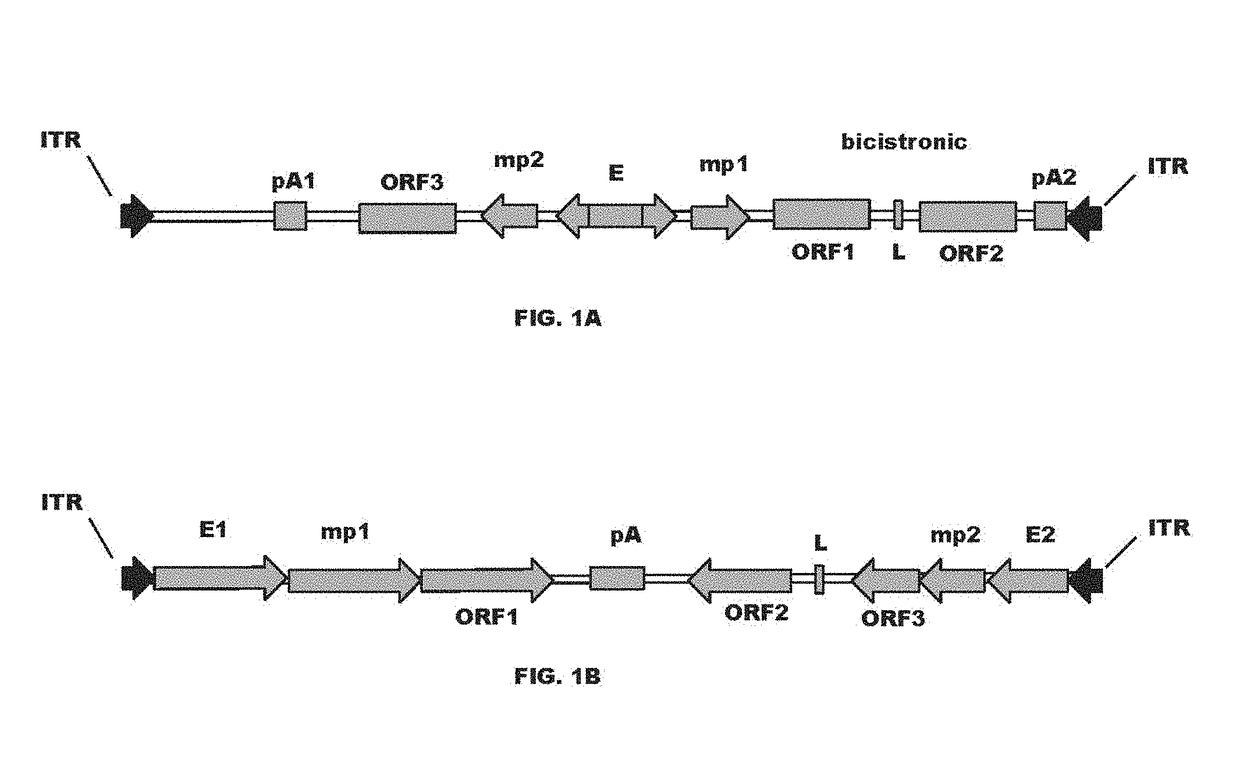
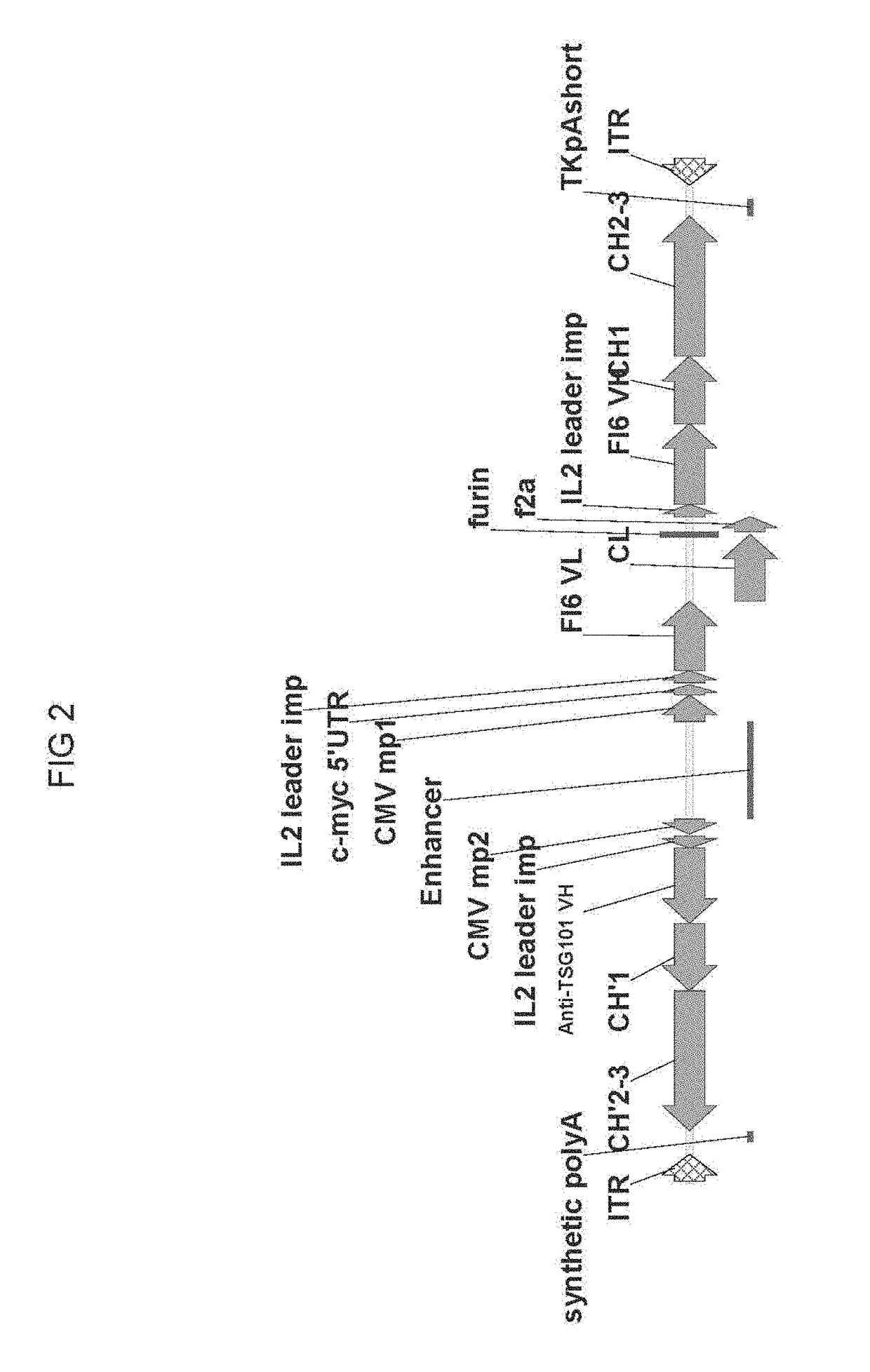
[0090]A series of cis-plasmids were prepared for use in generating an AAV viral particle containing a nucleic acid molecule for delivery to a host target cell. The nucleic acid molecules comprise AAV2 5′ and 3′ ITR sequences at each terminus, a shared CMV enhancer flanked by two expression cassettes in opposite orientations, where a first expression cassette is controlled by a first minimal CMV promoter and a second expression cassette is controlled by a second minimal CMV promoter. All sequences located between AAV2 ITRs were de novo synthesized by a commercial vendor (GeneArt). All coding sequences for immunoglobulin variable domains were flanked with the unique restriction enzymes to allow convenient shuttling of the desired variable domains. To create constructs with heterologous light chain sequence (kgl), a coding sequence encoding germline light chain (IGKV4-1*01) was de novo synthesized and used to replace F...
example 2
ization of Products Expressed from AAV8 Vectors Co-Expressing F16 Monoclonal Antibody (MAB) and IA6 MAB
[0095]A series of ELISA assays were performed to characterize expression levels and to assess binding of the FI6 MAB co-expressed with the IA6 MAB from the cis plasmid generated as described in Example 1 after transfection into HEK 293 cells. TSG101 peptide was synthesized using f-Moc chemistry by Mimotopes. All flu antigens were procured from a commercial supplier, ImmuneTechnologies, Inc. ProteinA was purchased from Sigma-Aldrich and was used to monitor expression of total human IgG1. Detection of human IgG1 in tissue culture supernatants was measured by either antigen-specific or proteinA capture ELISA. High binding ELISA plates were coated with 2 μg / ml of HA proteins or peptides, or with 5 μg / ml proteinA diluted in PBS and incubated overnight at 4° C. Wells were washed 5-8 times and blocked with 1 mM EDTA, 5% heat inactivated PBS, 0.07% Tween 20 in PBS for one hour at room temp...
example 3
ization of Products Expressed from AAV8 Vectors Co-Expressing Fi6 Monoclonal Antibody (MAB) and Pandemic Flu MAB C05
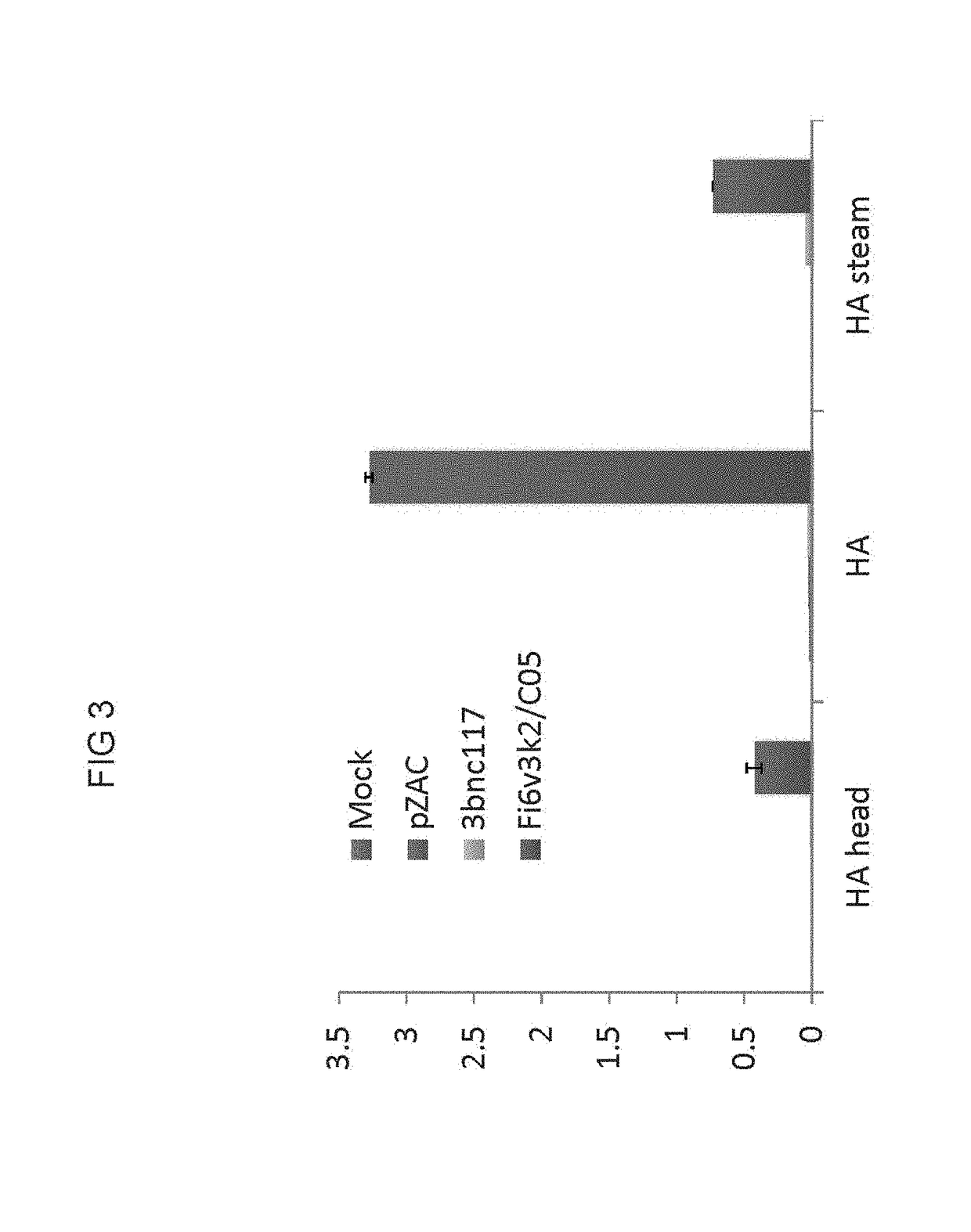
[0098]The possibility of differential detection of two different monoclonal antibodies was assessed in a capture assay. Monoclonal antibodies FI6 and C05 co-expressed from a cis-plasmid prepared as described in Example 1 and transfected into HEK293 cells were assessed for binding. FI6 is expected to bind to full-length HA and to HA stem, but not to the head only region. The results of the binding study illustrated in FIG. 3 demonstrate that the co-expressed antibodies retain their characteristic binding. More particularly, binding to full-length HA and the HA stem characteristic of FI6 is observed and binding to HA and HA head only (no stem) characteristic of C05 is also observed. ELISA assays were performed as described in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com