NON-CATALYTIC SUBSTRATE-SELECTIVE P38alpha-SPECIFIC MAPK INHIBITORS WITH ENDOTHELIAL-STABILIZING AND ANTI-INFLAMMATORY ACTIVITY, AND METHODS OF USE THEREOF
a technology of p38alpha-specific mapk inhibitors and substrates, which is applied in the field of compounds to achieve the effects of stabilizing the endothelial or epithelial barrier function, reducing inflammation, and reducing lps-induced lung injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ling of p38 MAPK Substrate-Docking Site, Compound Identification, and Screening Compounds for Direct, Selective Interaction with p38α
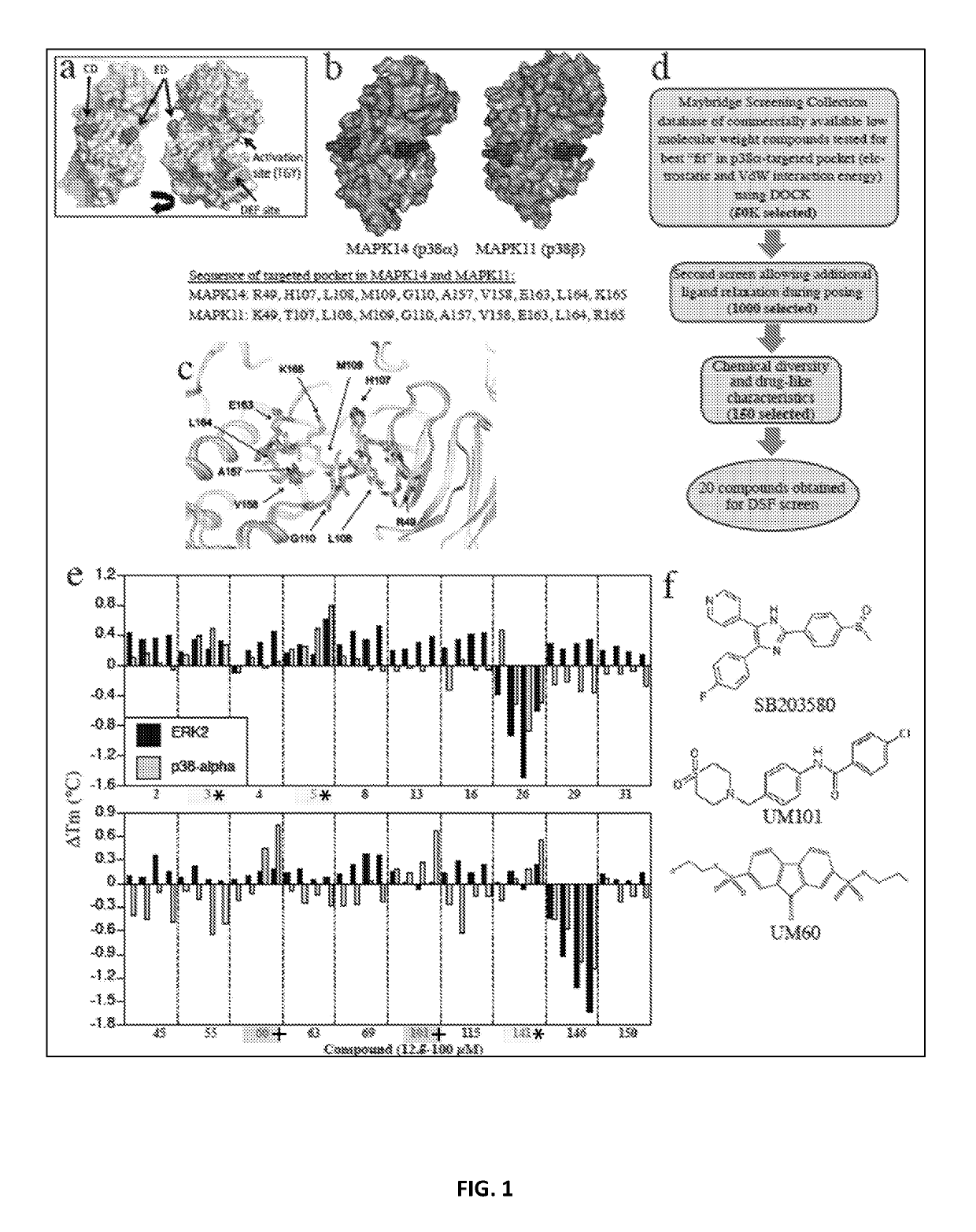
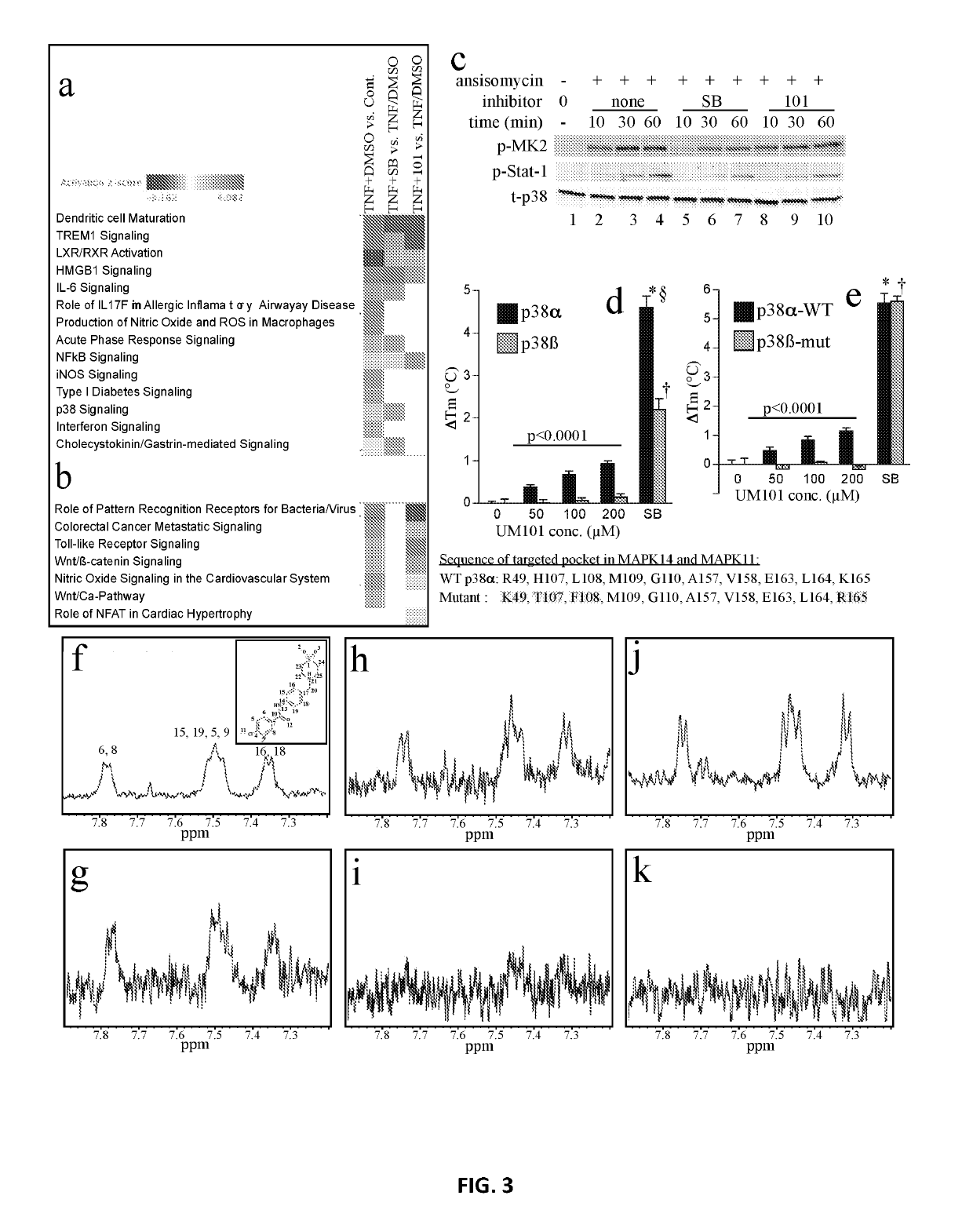
[0260]The inhibitors and methods of the invention relate to a CADD-based strategy to identify low molecular weight compounds predicted to bind near the ED substrate-docking site of mouse unphosphorylated p38α (MAPK14 variant-1; PDB:1P38), which is >99% identical with human p38α (variant-2) (FIG. 1a). The ED and CD sites in p38α are located at either end of a substrate-binding cleft located on the opposite side of the protein from the catalytic site (FIG. 1a). A pocket near the ED binding site comprising 10 amino acids, only 7 of which were identical in p38α and p38β, was identified (FIG. 1b). Overlay of structures of mouse unphosphorylated (PDB:1P38) and dual-phosphorylated p38α (PDB:3PY3) revealed near-superimposition of the targeted pocket in the two forms (FIG. 1c).
[0261]An overview of the CADD screening and compound testing protocols is shown in FI...
example 2
f Compounds on Endothelial Barrier Functions
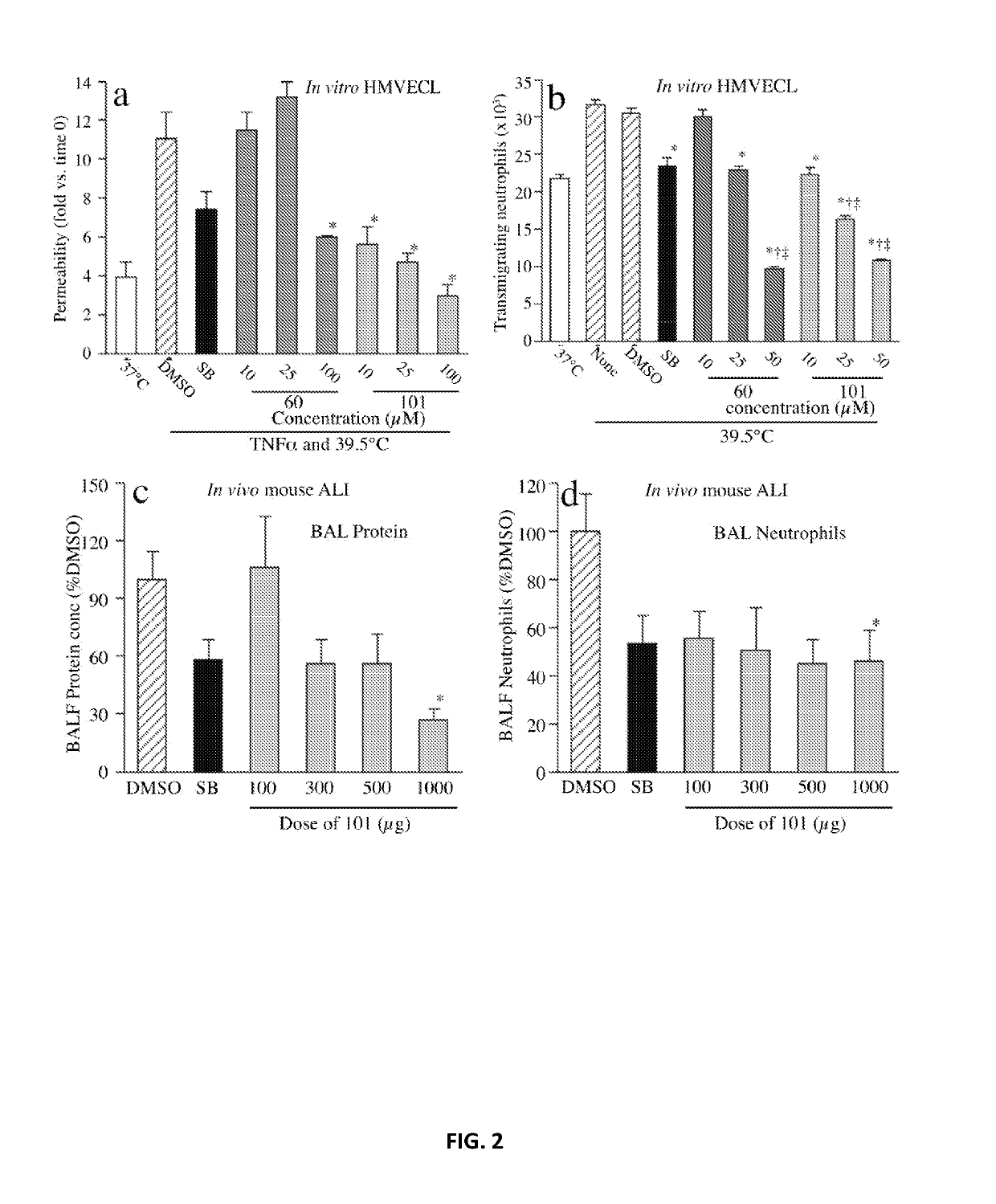
[0264]The capacity of UM60 and UM101 to stabilize endothelial barriers to macromolecules and neutrophils in TNFα- and hyperthermia-stressed HMVECL monolayers was tested (FIG. 2). Combined exposure to 1 ng / ml TNFα and hyperthermia (39.5° C.) for 6 h increased permeability for 10 kDa dextran 2.8-fold, compared with untreated 37° C. cells. Pretreating with 10 μM SB203580 for 30 min reduced TNFα / hyperthermia-induced permeability by 50% (FIG. 2a). Pretreatment with UM60 at 10 and 25 μM had no effect on permeability, but 100 μM UM60 reduced the TNFα / hyperthermia-induced permeability increase by 71% while UM101 at 10, 25, and 100 μM reduced the TNFα / hyperthermia-induced permeability increase by 74%, 89% and >100%, respectively.
[0265]Preincubating HMVECLs at 39.5° C. for 6 h increased subsequent IL-8-directed neutrophil TEM from 22.8±0.45×103 to 31.8±0.54×103 neutrophils (FIG. 2b). Pretreatment with 10 μM SB203580 reduced hyperthermia-augmented ne...
example 3
Effectiveness of SB203580 and UM101 in Mouse ALI
[0266]The effectiveness of UM60, UM101, and SB203580 in mitigating transalveolar protein and neutrophil extravasation in a mouse model of LPS / hyperthermia-induced ALI was compared (FIG. 2c and FIG. 2d). Mice received a single intraperitoneal injection of 100, 300, 500, or 1000 μg UM101, 1000 μg UM60, or 1000 μg SB203580 in 0.5 ml 2% DMSO 30 min prior to intratracheal instillation of 50 μg LPS and transfer to hyperthermic chambers. Control mice received DMSO. Four of six UM60-treated, one of six SB203580-treated, and one of eleven DMSO-treated control mice died within 24 h. All sixteen UM101-pretreated mice survived. Lung lavage from DMSO-pretreated, LPS / hyperthermia-challenged mice contained 1.09±0.19 mg / ml protein and 3.97±1.07×106 neutrophils. Compared with DMSO-treated controls, lavage protein concentration and neutrophil content in mice pretreated with 1000 μg SB203580 were reduced by 42% and 46.8%, respectively. Lavage protein con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com