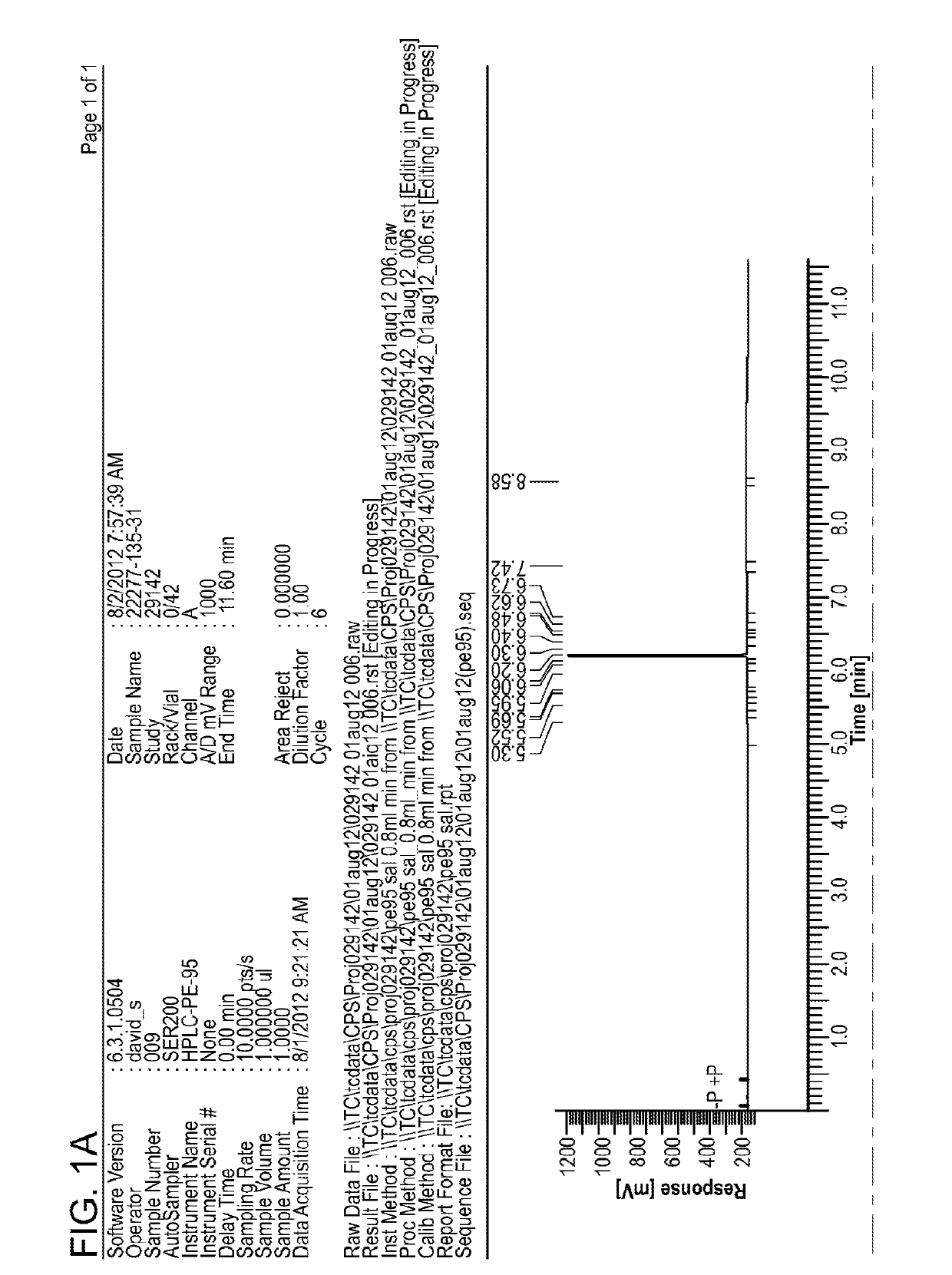
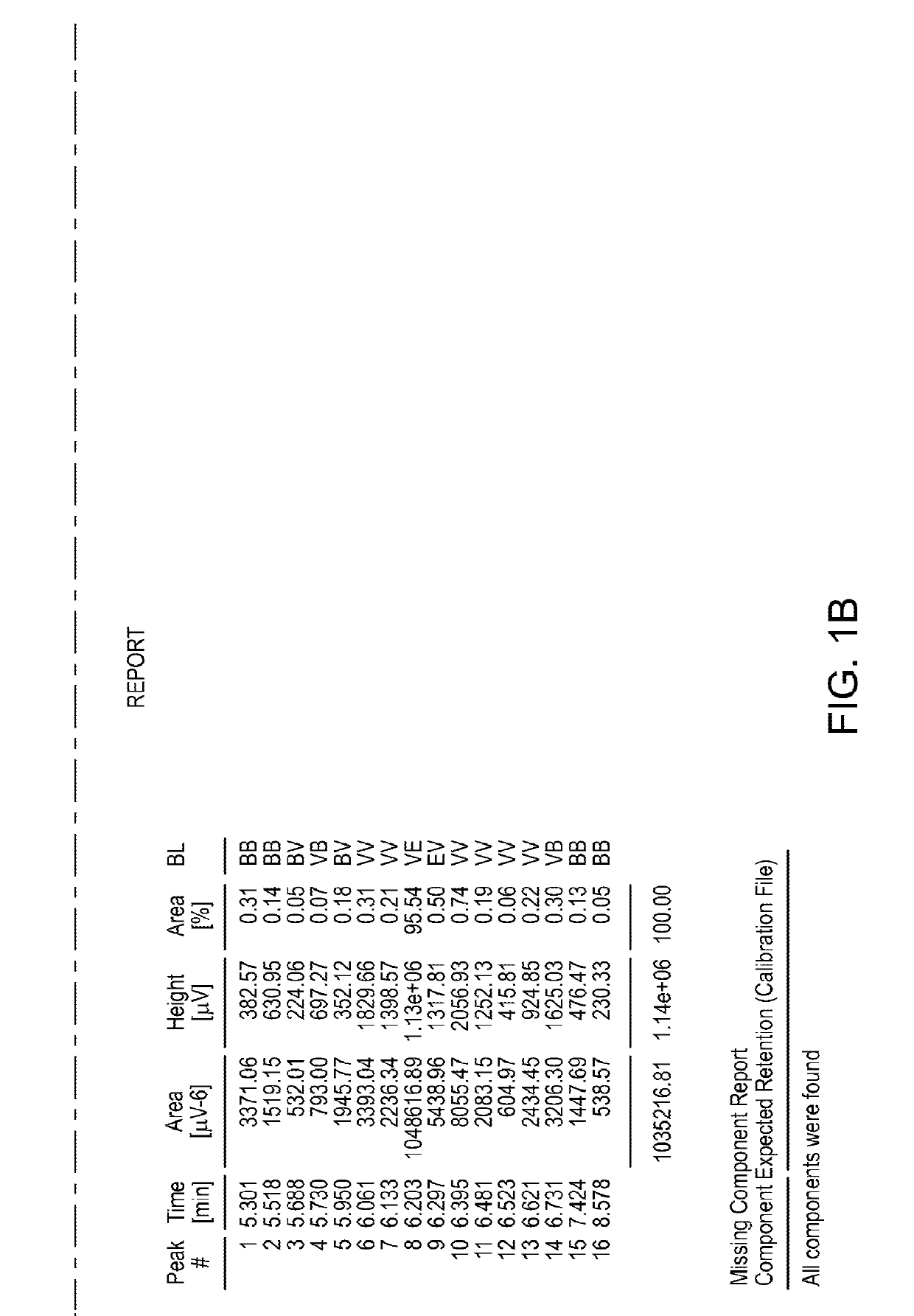
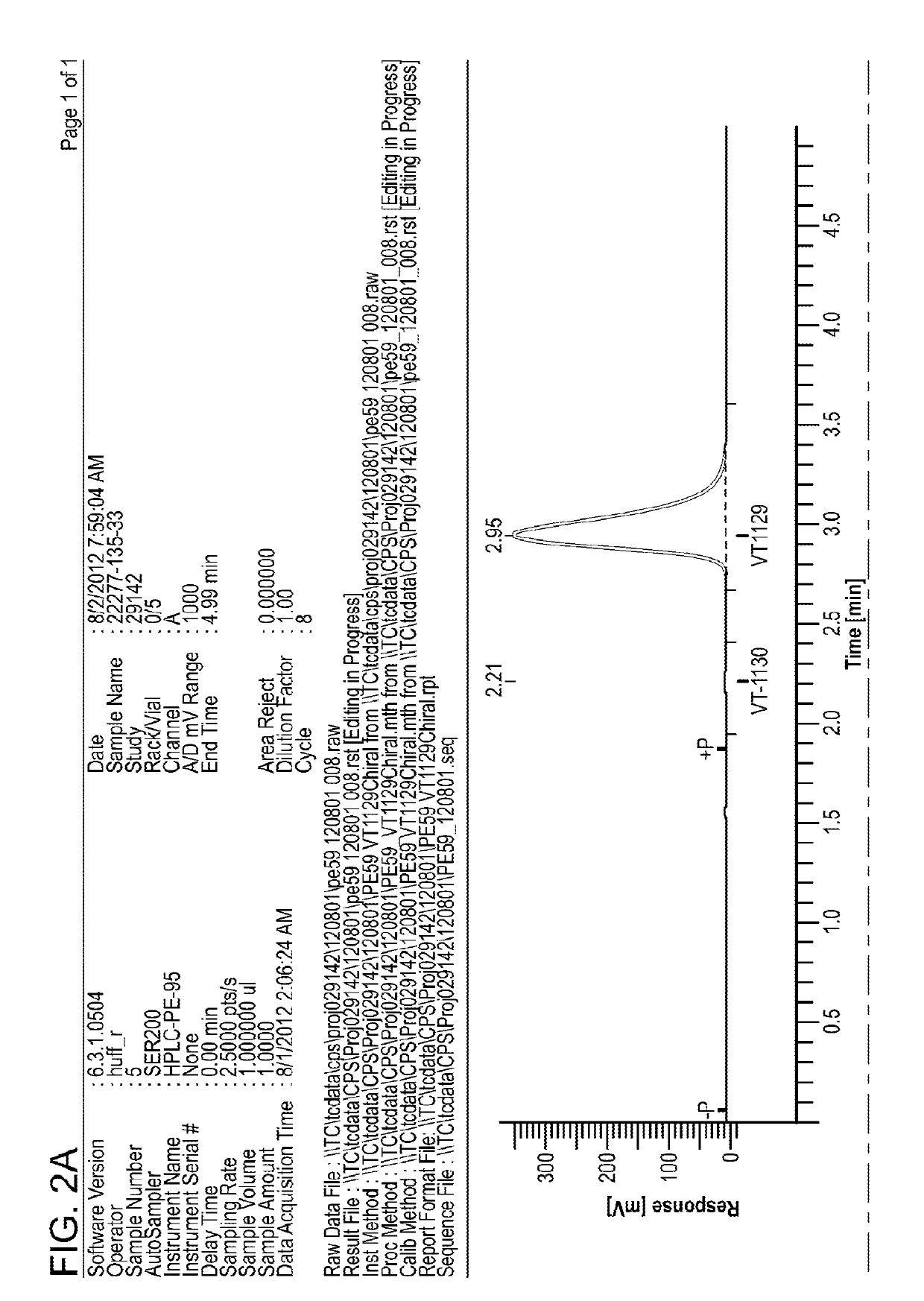
Antifungal Compound Process
a compound process and antifungal technology, applied in the field of antifungal compound process, can solve the problem of dramatic decrease of the activity of the enzym
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of 1-(2,4-difluorophenyl)-2,2-difluoro-2-(5-(4-(trifluoromethoxy)phenyl)pyridin-2-yl)ethanone (1-4)
1a. ethyl 2-(5-bromopyridin-2-yl)-2,2-difluoroacetate (2)
[0449]
[0450]Table 1 illustrates the effects of the relative proportions of each of the reagents and reactants, the effect of temperature, and the effect of varying the solvent had on the overall performance of the transformation as measured by the overall yield and purity of the reaction.
TABLE 1Process Development for the Preparation of compound 2-BrBr-esterCuSolventTempTime2-Br1Br-esterOtherEntry(eq1)(size / eq1)(vol)2(° C.)(h)(%)(%)(%)(%)11.043 μm / 2.5DMF (4)51847631121.043 μm / 2.5NMP (4)51841966631.04Cu bronzeDMF (4)5172078341.04Cu bronzeNMP (4)51721283351.043 μm / 2.5DMF (4)5148804761.023 μm / 2.4DMF (4)7520741.5971.023 μm / 1.0DMF (4)7517759481.023 μm / 1.5DMF (4)75178463691.023 μm / 2.0DMF (4)7517856100.9 3 μm / 2.16DMF (4)7517801116110.79 3 μm / 1.98DMF (4)751788416120.67 3 μm / 1.67DMF (4)75178675130.83 μm / 0.8DMF (4)751...
example 4
on of 2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-(5-(4-(trifluoromethoxy)phenyl)pyridin-2-yl)propan-2-ol (1 or 1a)
[0478]
[0479]The procedure used to generate compound 1 or 1a is as described in U.S. Pat. No. 4,426,531. Table 13 illustrates the efficient and quantitative nature of this procedure as performed on amino-alcohol 1-6* or 1-7* produced from both the TMS-cyanohydrin method and the TMSI-epoxidation method.
TABLE 13Formation of Compound 1 or 1a1-6* or 1-7*Cmpd 1 or 1a1-6* or 1-7*1-6* or 1-7*PurityPurityYieldEntryOriginAmt (g)(HPLC %)(HPLC %)(%)1TMSI-199.097.496.5epoxidationMethod2TMS-198.097.999.2cyanohydrinMethod
example 5
fluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-(5-(4-(trifluoromethoxy)phenyl)pyridin-2-yl)propan-2-ol benzenesulfonate (1 or 1a-BSA)
[0480]
Typical Procedure for Converting 1 or 1a to 1 or 1a-BSA
[0481]46.6 g of compound 1 or 1a was dissolved in ethylacetate (360 ml). The solution was filtered through a glass microfiber filter and placed in a 2 L reaction flask equipped with an overhead stirrer, condenser, and a J-Kem thermocouple. Pharma-grade benzenesulfonic acid (BSA, 14.39 g, 1 eq) was dissolved in ethyl acetate (100 ml). The BSA solution was filtered through a glass microfiber filter and added to the stirred 1 or 1a solution in one portion. The mixture was warmed to 60-65° C.; precipitation of the 1 or 1a / BSA salt occurred during the warm up period. The slurry was held for 60 minutes at 60-65° C. The suspension was allowed to slowly cool to 22° C. and was stirred at 20-25° C. for 16 hours. n-Heptane (920 ml) was charged in one portion and the suspension was stirred at 22° C. f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com