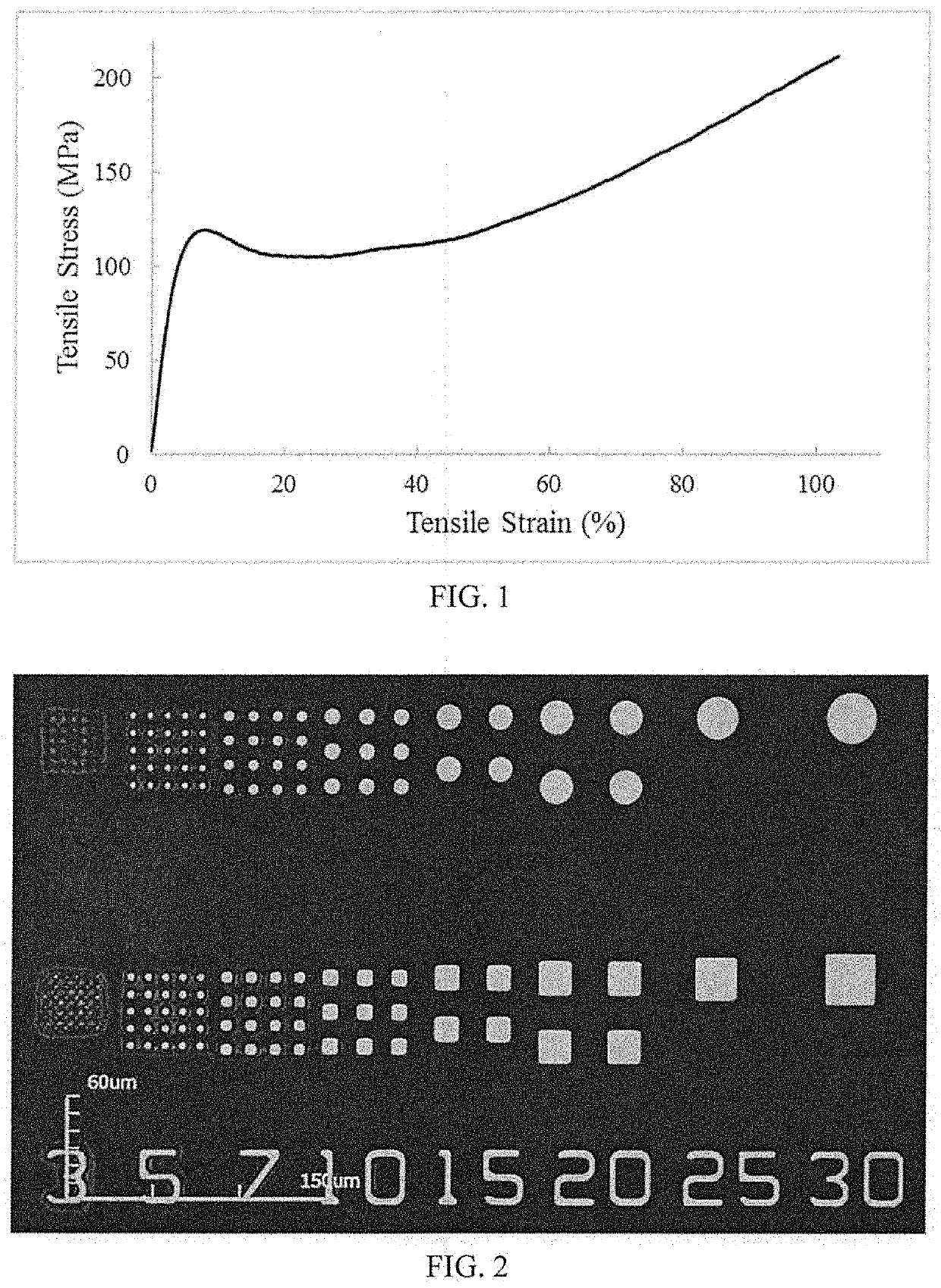
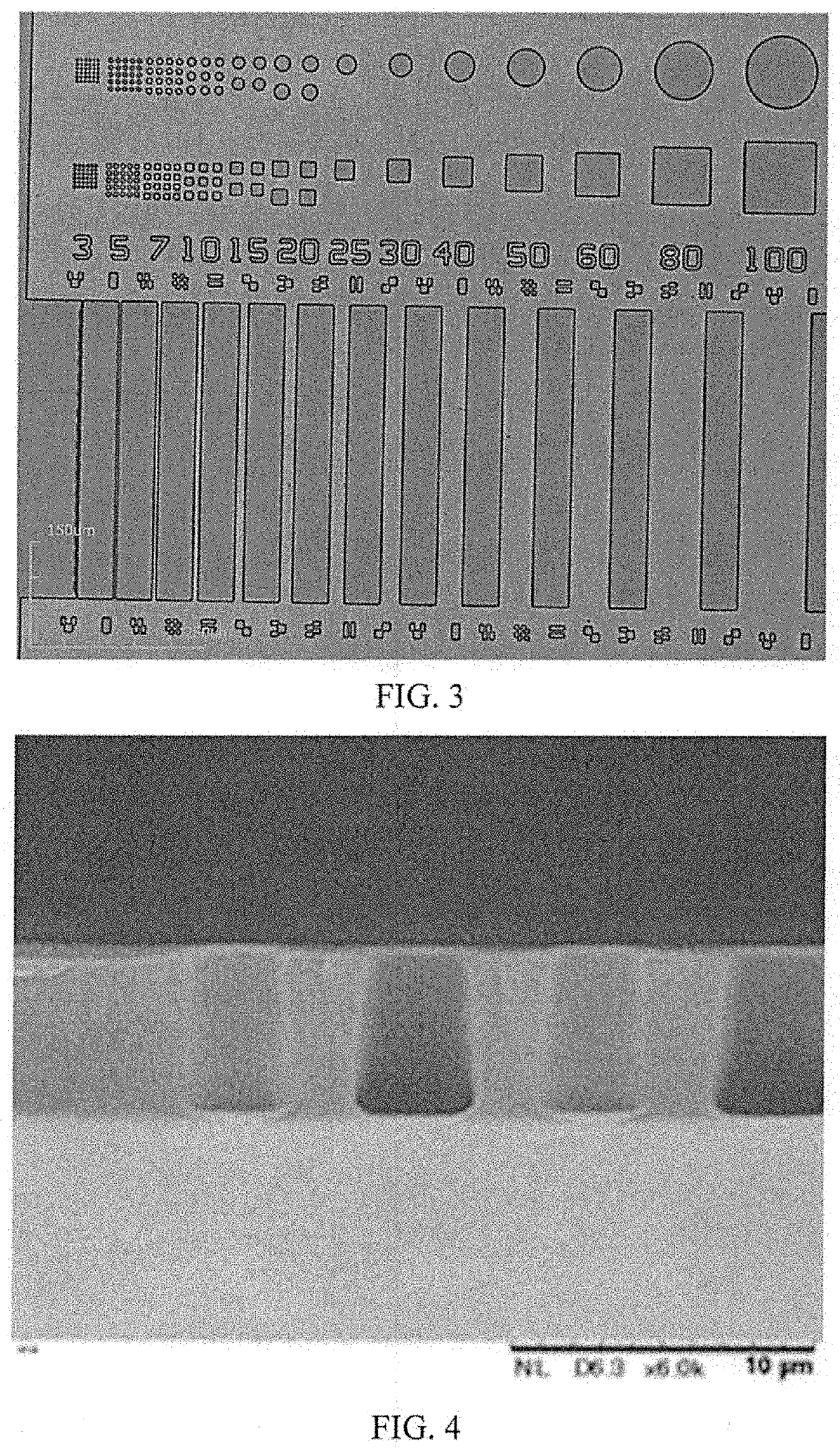
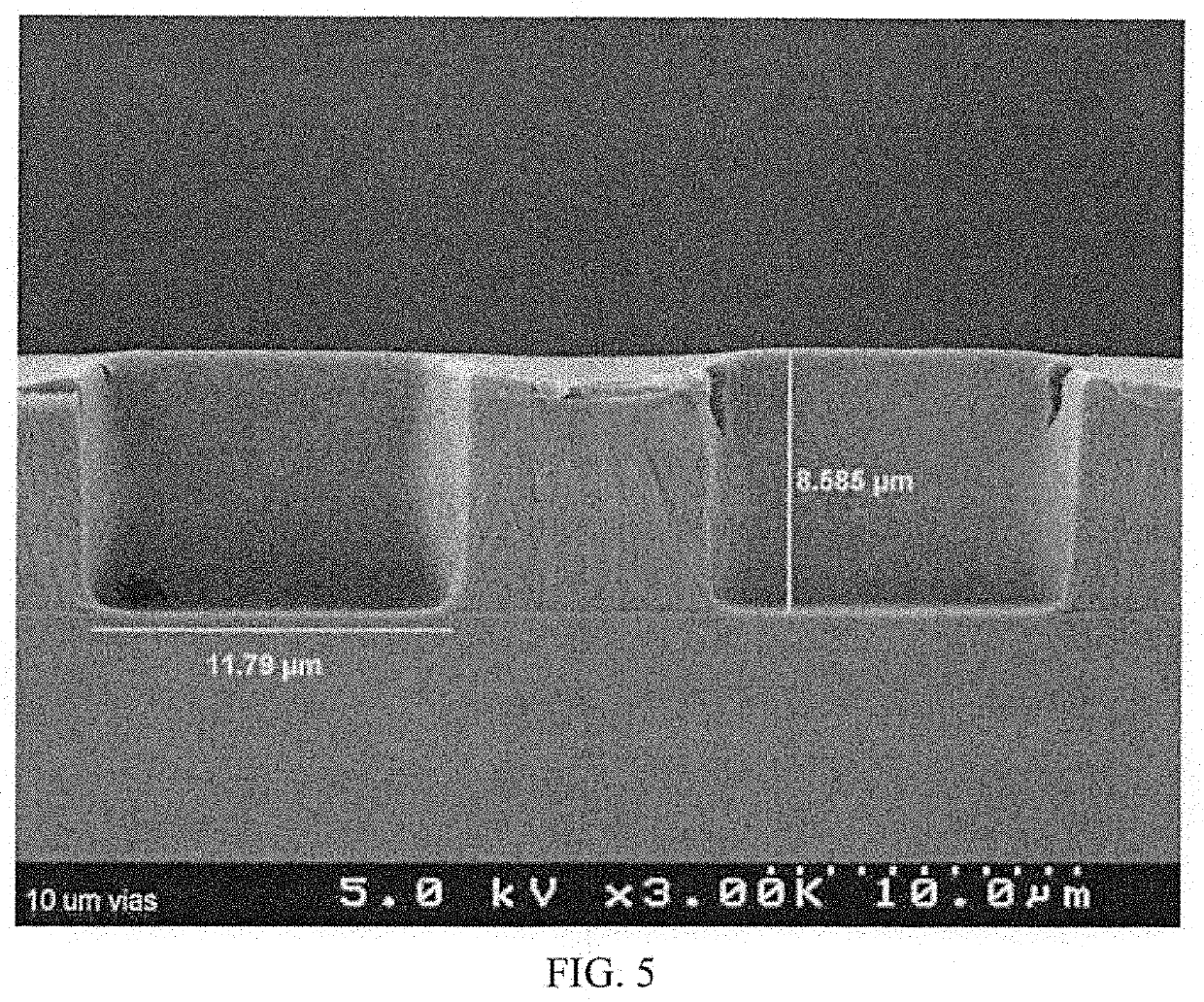
Photosensitive polyimide compositions
a polyimide and composition technology, applied in the field of photosensitive polyimide compositions, can solve the problems of poor photo imaging capability, insolubility of polyimides and/or precursor polyamic acids in commonly used solvents in the electronic industry, and many not suitable for many applications, and achieve excellent thermomechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6FDA / PMDA / 6BF / PFMB (25 / 25 / 30 / 20)
[0209]6BF (15.13 g, 30 mmol) and PFMB (6.41 g, 20 mmol) were dissolved in NMP (152.4 g) and stirred at ambient temperature under a nitrogen atmosphere. To this solution was then added a mixture of 6FDA (11.11 g, 25 mmol) and PMDA (5.45 g, 25 mmol) in small batches while stirring that generated about 5° C. exotherm. After which time the reaction mixture was continued to stir at ambient temperature for 20 hours during which time the solution turned viscous indicating the formation of polyamic acid. To this viscous solution additional amount of NMP (184 g) was added and a small portion of which was characterized by GPC (GPC-DMAc—Mw=258,950, Mn=124,450, PDI=2.08).
[0210]The polyamic acid solution as obtained above (236 g containing 23.6 g polymer) was mixed with anhydrous pyridine (23.9 g), acetic anhydride (24 g) and cyclopentanone (60.6 g) and the solution heated to 95° C. for 4 hours under nitrogen atmosphere while stirring. The reaction mixture was all...
example 2
6FDA / PMDA / 6BF / PFMB / DMMIEt-NH2 (24.7 / 24.7 / 29 / 19.1 / 2.5)
[0211]6BF (14.82 g, 29.4 mmol), PFMB (6.21 g, 19.4 mmol) and DMMIEt-NH2 (0.42 g, 2.5 mmol) were dissolved in NMP (152 g) and stirred at ambient temperature under a nitrogen atmosphere. To this solution was then added a mixture of 6FDA (11.11 g, 25 mmol) and PMDA (5.45 g, 25 mmol) in small batches while stirring. The reaction mixture was stirred at ambient temperature for 20 hours during which time the solution became viscous. To this viscous solution was then added NMP (72 g). A small portion of this solution was further diluted with DMAc for GPC analysis. GPC-DMAc—Mw=131,500, Mn=72,550, PDI=1.81. A small sample of the polymer solution was added to excess water / acetone (80 / 20) mixture to isolate the polymer. The gummy product was washed with excess water / acetone (80 / 20) mixture and dried in a vacuum oven at 50-60° C. for 24 hours to obtain a solid product, which was characterized by 1H NMR. 1H-NMR (500 MHz) spectra of this product...
example 3
6FDA / PMDA / 6BF / PFMB / DMMIEt-NH2 (24.7 / 24.7 / 29.0 / 19.1 / 2.5)
[0213]6BF (17.78 g, 35.3 mmol), PFMB (7.45 g, 23.3 mmol) and DMMIEt-NH2 (0.51 g, 3 mmol) were dissolved in NMP (182.4 g) and stirred at ambient temperature under a nitrogen atmosphere. A mixture of 6FDA (13.33 g, 30 mmol) and PMDA (6.54 g, 30 mmol) was then added in small batches to the above solution while stirring. The reaction mixture was stirred at ambient temperature for 20 hours during which time the solution turned viscous. An additional amount of NMP (62 g) was added to this viscous solution. A small portion of this solution was further diluted with DMAc for GPC analysis. GPC-DMAc—Mw=121,150, Mn=69,500, PDI=1.74. A small sample of this polymer solution was also added to excess water / acetone (80 / 20) mixture to isolate the polymer. The gummy product was washed with excess water / acetone (80 / 20) mixture and dried in a vacuum oven at 50-60° C. for 24 hours to obtain a solid product, which was characterized by 1H NMR. 1H-NMR (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com